Please answer the questions
1)Mechanism (if applicable, must be handwritten, never typed): Write clear stepwise mechanisms for all synthetic transformations, showing important intermediates where appropriate.
2)Flowchart of Procedure (6 pts) (must be handwritten, never typed) Provide a diagram or scheme of the procedural steps you will be conducting (see page p. 23 of your lab manual for an example of a flowchart). Be sure to include specific information such as chemical names, quantities, temperatures, time frames, etc. You should not transcribe the manual word for word; sketch it out, make notes to yourself, and use pictures and abbreviations. You should also write a list of glassware and chemicals you'll need for the experiment, and draw your glassware setup(s).
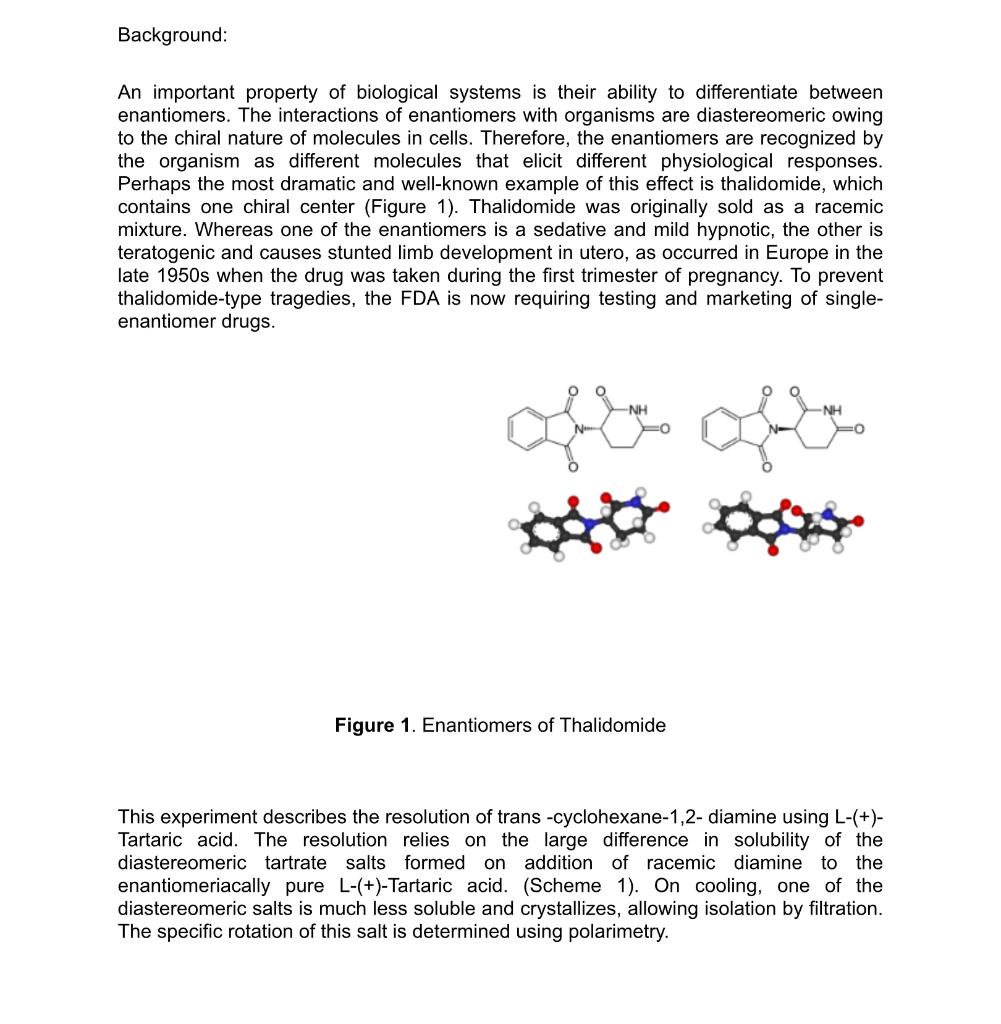
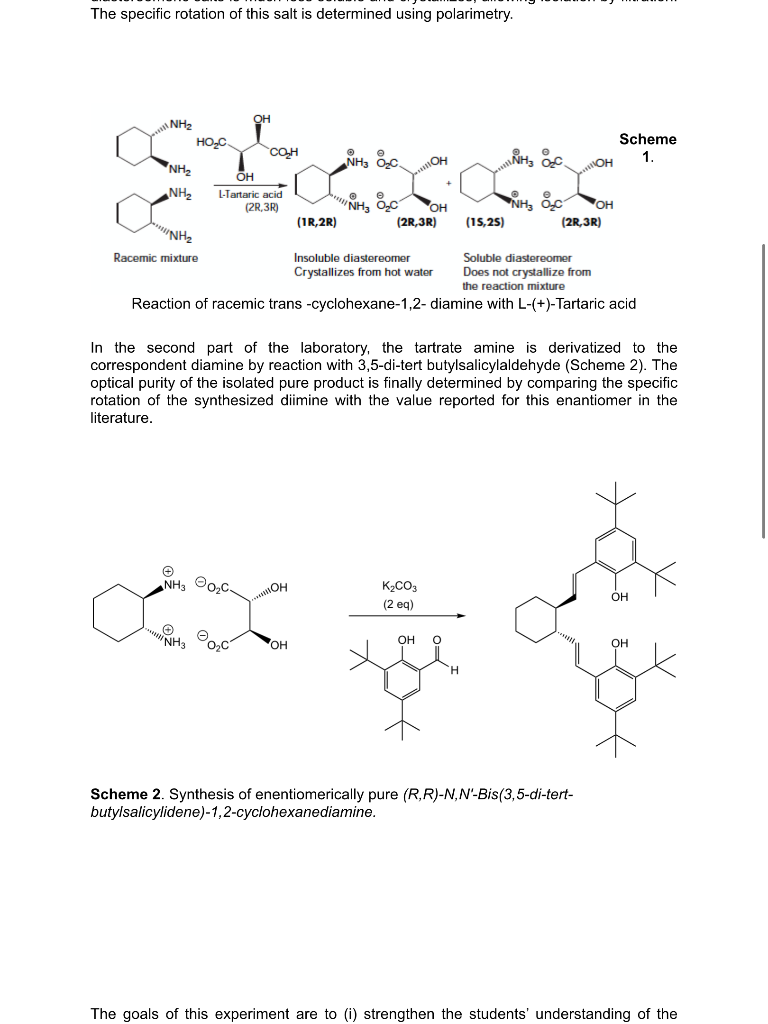


Background: An important property of biological systems is their ability to differentiate between enantiomers. The interactions of enantiomers with organisms are diastereomeric owing to the chiral nature of molecules in cells. Therefore, the enantiomers are recognized by the organism as different molecules that elicit different physiological responses. Perhaps the most dramatic and well-known example of this effect is thalidomide, which contains one chiral center (Figure 1). Thalidomide was originally sold as a racemic mixture. Whereas one of the enantiomers is a sedative and mild hypnotic, the other is teratogenic and causes stunted limb development in utero, as occurred in Europe in the late 1950s when the drug was taken during the first trimester of pregnancy. To prevent thalidomide-type tragedies, the FDA is now requiring testing and marketing of singleenantiomer drugs. Figure 1. Enantiomers of Thalidomide This experiment describes the resolution of trans -cyclohexane-1,2- diamine using L-(+)Tartaric acid. The resolution relies on the large difference in solubility of the diastereomeric tartrate salts formed on addition of racemic diamine to the enantiomeriacally pure L-(+)-Tartaric acid. (Scheme 1). On cooling, one of the diastereomeric salts is much less soluble and crystallizes, allowing isolation by filtration. The specific rotation of this salt is determined using polarimetry. The specific rotation of this salt is determined using polarimetry. Reaction of racemic trans -cyclohexane-1,2- diamine with L-(+)-Tartaric acid In the second part of the laboratory, the tartrate amine is derivatized to the correspondent diamine by reaction with 3,5-di-tert butylsalicylaldehyde (Scheme 2). The optical purity of the isolated pure product is finally determined by comparing the specific rotation of the synthesized dimine with the value reported for this enantiomer in the literature. Scheme 2. Synthesis of enentiomerically pure (R,R)N,NBis(3,5-di-tertbutylsalicylidene)-1,2-cyclohexanediamine. The goals of this experiment are to (i) strengthen the students' understanding of the The goals of this experiment are to (i) strengthen the students' understanding of the relationship between enantiomers and diastereomers and explain how the differences in properties of diastereomers can be used in a resolution, (ii) illustrate the difference in physical properties (solubility and melting range) between a racemate and the same compound of high optical purity, (iii) provide experience in an important technique for determining optical purity (polarimetry). Step 1. Preparation and Recrystallization of a Diastereomeric Salt - Synthesis of (R,R)1,2-Diammoniumcyclohexane mono-(+)-tartrate: In a 150mL beaker, L-(+)-Tartaric acid (7.5g,0.05mol) is dissolved in 25mL of distilled water. The solution is stirred as 11.4g(12.2mL,0.10mol) of 1,2-diaminocyclohexane is added carefully in one portion. (The addition of the diamine is exothermic.) A slurry is formed initially but complete dissolution is observed once addition is complete. After a few minutes, 5.0mL of glacial acetic acid is added in one portion. Product begins to fall out of solution shortly after the addition, and it continues to precipitate while the reaction mixture is cooled to 5C in an ice/water bath. After sitting for at least 30 minutes in an ice bath at 5C, the product is isolated by filtration. The filter cake is washed with 5.0 mL of cold (5C) water followed by 45mL portions of room temperature methanol. a) While you wait for the solid to precipitate, your instructor will demonstrate how to use the polarimeter by measuring the optical rotation of racemic 1,2-diamino cyclohexane and of (1R,2R)()1,2-Diaminocyclohexane. Record the results in your notebook and discuss it in your post-lab report. Step 2. Preparation of Jacobsen's Ligand: Formation of a Diimine (R,R)N,N-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine. A 100mL, round bottomed flask equipped with a magnetic stir bar is charged with 6.0 mL of water, 1.11g of (R,R)-1,2-diaminocyclohexane mono-(+)-tartrate salt, and 1.16 grams of potassium carbonate. The mixture is stirred until dissolution is achieved, then 22mL of ethanol are added. The flask is equipped with a reflux condenser, and the cloudy mixture is heated to reflux with a heating mantle. A solution of 2.0g of 3,5-di-tert butylsalicylaldehyde dissolved in 10mL of ethanol (this requires heating carefully on a hot plate) is added through the condenser with a Pasteur pipet. The pipet and beaker are then rinsed with 2.0mL of hot ethanol. After heating at reflux for one hour, heating is discontinued. Water (6.0mL) is added and the mixture is cooled to 5C and left to stand at that temperature for 30 minutes. The yellow solid is collected by vacuum filtration and washed with 4-5 mL of ethanol. The solid is dissolved in 25mL of methylene chloride, and the solution is washed with 25mL of water, followed by 5mL saturated aqueous NaCl. The organic layer is dried over Na2SO4, then filtered and the sample is left drying in the fume hood for a week. During the next laboratory session you will measure the melting point of your solid and the optical activity of the sample ([a]D) cloudy mixture is heated to reflux with a heating mantle. A solution of 2.0g of 3,5 -di-tert butylsalicylaldehyde dissolved in 10mL of ethanol (this requires heating carefully on a hot plate) is added through the condenser with a Pasteur pipet. The pipet and beaker are then rinsed with 2.0mL of hot ethanol. After heating at reflux for one hour, heating is discontinued. Water (6.0mL) is added and the mixture is cooled to 5C and left to stand at that temperature for 30 minutes. The yellow solid is collected by vacuum filtration and washed with 4-5 mL of ethanol. The solid is dissolved in 25mL of methylene chloride, and the solution is washed with 25mL of water, followed by 5mL saturated aqueous NaCl. The organic layer is dried over Na2SO4, then filtered and the sample is left drying in the fume hood for a week. During the next laboratory session you will measure the melting point of your solid and the optical activity of the sample ([[D) Analysis of the Resolution of 1,2-Diaminocyclohexane. In order to determine the success of your resolution of 1,2-diaminocyclohexane, you will measure the optical activity of your (R,R)N,N '-bis(3,5-di-tert-butylsalicylidene)-1,2cyclohexanediamine. (The Jacobsen ligand that you prepared above.) Weigh accurately 0.5-1.0 g of your Jacobsen ligand and dissolve it in CH2Cl2 to make a final volume of 10 mL (use a volumetric flask). Determine the optical rotation of this solution. (Your instructor will discuss the care and feeding of the polarimeter during the previous lab session) Calculate the specific rotation for your sample and compare it to the reported value: [a]D20=315(c=1,CH2Cl2). Post-Lab Report Questions: 1) Draw the structure of 1R,2R)()1,2-Diaminocyclohexane 2) Discuss the optical rotation value obtained for both racemic 1,2-diamino cyclohexane and (1R,2R)()1,2-Diaminocyclohexane. 3) Predict the optical rotation value of (1S,2S)-1,2-Diaminocyclohexane. 4) Are the melting points of racemic 1,2-diamino cyclohexane and (1R,2R)()1,2 Diaminocyclohexane the same ? Explain. 5) Define enantiomeric excess. Calculate it for the synthesized dimine (Jacob's ligand). 6) Was the resolution of racemic 1,2-Diaminocyclohexane successful? Explain. References: 1. Larrow, J.F.; Jacobsen, E.N. Organic Syntheses, Vol. 75; Wiley: New York, 1998; pp 1-11. 2. Walsh, P.J.; Smith, D.K.; Castello, C. J. Chem. Ed. 1998, 75, 1459. Background: An important property of biological systems is their ability to differentiate between enantiomers. The interactions of enantiomers with organisms are diastereomeric owing to the chiral nature of molecules in cells. Therefore, the enantiomers are recognized by the organism as different molecules that elicit different physiological responses. Perhaps the most dramatic and well-known example of this effect is thalidomide, which contains one chiral center (Figure 1). Thalidomide was originally sold as a racemic mixture. Whereas one of the enantiomers is a sedative and mild hypnotic, the other is teratogenic and causes stunted limb development in utero, as occurred in Europe in the late 1950s when the drug was taken during the first trimester of pregnancy. To prevent thalidomide-type tragedies, the FDA is now requiring testing and marketing of singleenantiomer drugs. Figure 1. Enantiomers of Thalidomide This experiment describes the resolution of trans -cyclohexane-1,2- diamine using L-(+)Tartaric acid. The resolution relies on the large difference in solubility of the diastereomeric tartrate salts formed on addition of racemic diamine to the enantiomeriacally pure L-(+)-Tartaric acid. (Scheme 1). On cooling, one of the diastereomeric salts is much less soluble and crystallizes, allowing isolation by filtration. The specific rotation of this salt is determined using polarimetry. The specific rotation of this salt is determined using polarimetry. Reaction of racemic trans -cyclohexane-1,2- diamine with L-(+)-Tartaric acid In the second part of the laboratory, the tartrate amine is derivatized to the correspondent diamine by reaction with 3,5-di-tert butylsalicylaldehyde (Scheme 2). The optical purity of the isolated pure product is finally determined by comparing the specific rotation of the synthesized dimine with the value reported for this enantiomer in the literature. Scheme 2. Synthesis of enentiomerically pure (R,R)N,NBis(3,5-di-tertbutylsalicylidene)-1,2-cyclohexanediamine. The goals of this experiment are to (i) strengthen the students' understanding of the The goals of this experiment are to (i) strengthen the students' understanding of the relationship between enantiomers and diastereomers and explain how the differences in properties of diastereomers can be used in a resolution, (ii) illustrate the difference in physical properties (solubility and melting range) between a racemate and the same compound of high optical purity, (iii) provide experience in an important technique for determining optical purity (polarimetry). Step 1. Preparation and Recrystallization of a Diastereomeric Salt - Synthesis of (R,R)1,2-Diammoniumcyclohexane mono-(+)-tartrate: In a 150mL beaker, L-(+)-Tartaric acid (7.5g,0.05mol) is dissolved in 25mL of distilled water. The solution is stirred as 11.4g(12.2mL,0.10mol) of 1,2-diaminocyclohexane is added carefully in one portion. (The addition of the diamine is exothermic.) A slurry is formed initially but complete dissolution is observed once addition is complete. After a few minutes, 5.0mL of glacial acetic acid is added in one portion. Product begins to fall out of solution shortly after the addition, and it continues to precipitate while the reaction mixture is cooled to 5C in an ice/water bath. After sitting for at least 30 minutes in an ice bath at 5C, the product is isolated by filtration. The filter cake is washed with 5.0 mL of cold (5C) water followed by 45mL portions of room temperature methanol. a) While you wait for the solid to precipitate, your instructor will demonstrate how to use the polarimeter by measuring the optical rotation of racemic 1,2-diamino cyclohexane and of (1R,2R)()1,2-Diaminocyclohexane. Record the results in your notebook and discuss it in your post-lab report. Step 2. Preparation of Jacobsen's Ligand: Formation of a Diimine (R,R)N,N-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine. A 100mL, round bottomed flask equipped with a magnetic stir bar is charged with 6.0 mL of water, 1.11g of (R,R)-1,2-diaminocyclohexane mono-(+)-tartrate salt, and 1.16 grams of potassium carbonate. The mixture is stirred until dissolution is achieved, then 22mL of ethanol are added. The flask is equipped with a reflux condenser, and the cloudy mixture is heated to reflux with a heating mantle. A solution of 2.0g of 3,5-di-tert butylsalicylaldehyde dissolved in 10mL of ethanol (this requires heating carefully on a hot plate) is added through the condenser with a Pasteur pipet. The pipet and beaker are then rinsed with 2.0mL of hot ethanol. After heating at reflux for one hour, heating is discontinued. Water (6.0mL) is added and the mixture is cooled to 5C and left to stand at that temperature for 30 minutes. The yellow solid is collected by vacuum filtration and washed with 4-5 mL of ethanol. The solid is dissolved in 25mL of methylene chloride, and the solution is washed with 25mL of water, followed by 5mL saturated aqueous NaCl. The organic layer is dried over Na2SO4, then filtered and the sample is left drying in the fume hood for a week. During the next laboratory session you will measure the melting point of your solid and the optical activity of the sample ([a]D) cloudy mixture is heated to reflux with a heating mantle. A solution of 2.0g of 3,5 -di-tert butylsalicylaldehyde dissolved in 10mL of ethanol (this requires heating carefully on a hot plate) is added through the condenser with a Pasteur pipet. The pipet and beaker are then rinsed with 2.0mL of hot ethanol. After heating at reflux for one hour, heating is discontinued. Water (6.0mL) is added and the mixture is cooled to 5C and left to stand at that temperature for 30 minutes. The yellow solid is collected by vacuum filtration and washed with 4-5 mL of ethanol. The solid is dissolved in 25mL of methylene chloride, and the solution is washed with 25mL of water, followed by 5mL saturated aqueous NaCl. The organic layer is dried over Na2SO4, then filtered and the sample is left drying in the fume hood for a week. During the next laboratory session you will measure the melting point of your solid and the optical activity of the sample ([[D) Analysis of the Resolution of 1,2-Diaminocyclohexane. In order to determine the success of your resolution of 1,2-diaminocyclohexane, you will measure the optical activity of your (R,R)N,N '-bis(3,5-di-tert-butylsalicylidene)-1,2cyclohexanediamine. (The Jacobsen ligand that you prepared above.) Weigh accurately 0.5-1.0 g of your Jacobsen ligand and dissolve it in CH2Cl2 to make a final volume of 10 mL (use a volumetric flask). Determine the optical rotation of this solution. (Your instructor will discuss the care and feeding of the polarimeter during the previous lab session) Calculate the specific rotation for your sample and compare it to the reported value: [a]D20=315(c=1,CH2Cl2). Post-Lab Report Questions: 1) Draw the structure of 1R,2R)()1,2-Diaminocyclohexane 2) Discuss the optical rotation value obtained for both racemic 1,2-diamino cyclohexane and (1R,2R)()1,2-Diaminocyclohexane. 3) Predict the optical rotation value of (1S,2S)-1,2-Diaminocyclohexane. 4) Are the melting points of racemic 1,2-diamino cyclohexane and (1R,2R)()1,2 Diaminocyclohexane the same ? Explain. 5) Define enantiomeric excess. Calculate it for the synthesized dimine (Jacob's ligand). 6) Was the resolution of racemic 1,2-Diaminocyclohexane successful? Explain. References: 1. Larrow, J.F.; Jacobsen, E.N. Organic Syntheses, Vol. 75; Wiley: New York, 1998; pp 1-11. 2. Walsh, P.J.; Smith, D.K.; Castello, C. J. Chem. Ed. 1998, 75, 1459










