Question
The ideal gas law relates the pressure P, volume V, and temperature T of an ideal gas: PV = nRT where n is the
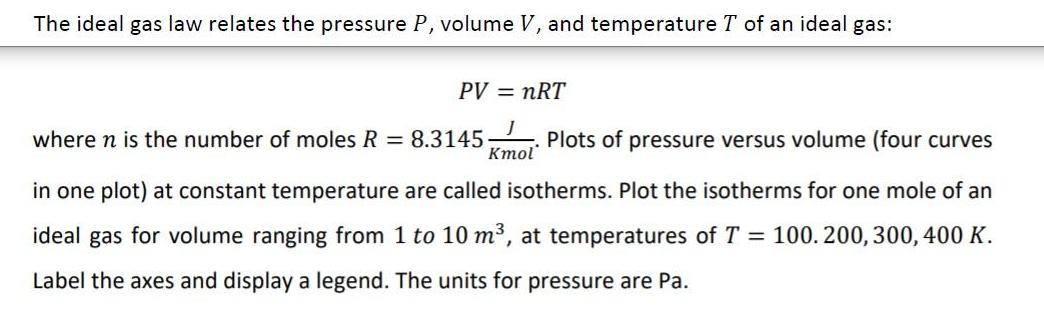
The ideal gas law relates the pressure P, volume V, and temperature T of an ideal gas: PV = nRT where n is the number of moles R = 8.31145 Plots of pressure versus volume (four curves " in one plot) at constant temperature are called isotherms. Plot the isotherms for one mole of an ideal gas for volume ranging from 1 to 10 m, at temperatures of T = 100. 200, 300, 400 K. Label the axes and display a legend. The units for pressure are Pa.
Step by Step Solution
3.52 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Matlab An Introduction with Applications
Authors: Amos Gilat
5th edition
1118629868, 978-1118801802, 1118801806, 978-1118629864
Students also viewed these Programming questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



