Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Which of the following relationships is correct at constant T and P? A. AG is proportional to -ASuniv B. AG < 0 represents a
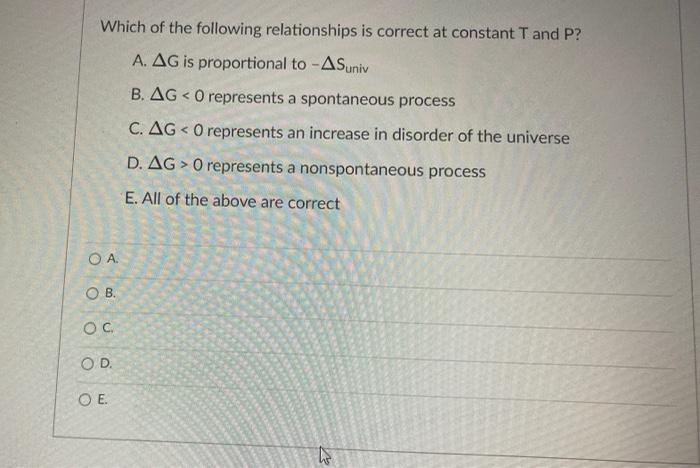

Which of the following relationships is correct at constant T and P? A. AG is proportional to -ASuniv B. AG < 0 represents a spontaneous process C. AG < 0 represents an increase in disorder of the universe D. AG> 0 represents a nonspontaneous process E. All of the above are correct OA. OB. OC OD. O E. If a reaction is exothermic and results in decreased entropy, what is true about the reaction? A. This reaction will be spontaneous only at low temperatures. B. This reaction will be spontaneous at all temperatures. C. This reaction will be nonspontaneous at all temperatures. D. This reaction will be nonspontaneous only at low temperatures. E. It is not possible to determine without more information. OA. OB. OC OD. OE
Step by Step Solution
★★★★★
3.49 Rating (172 Votes )
There are 3 Steps involved in it
Step: 1
Answers A AG is proportional to ASuniv B AGO represents a spontaneous process D AG 0 represents a nonspontaneous process A This reaction will be spontaneous only at low temperatures Explanation 1 Answ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


