Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer, will upvote! 1. A student reacted phenethyl alcohol with PCC (shown below) but wasn't sure what the product of the reaction was going
please answer, will upvote! 
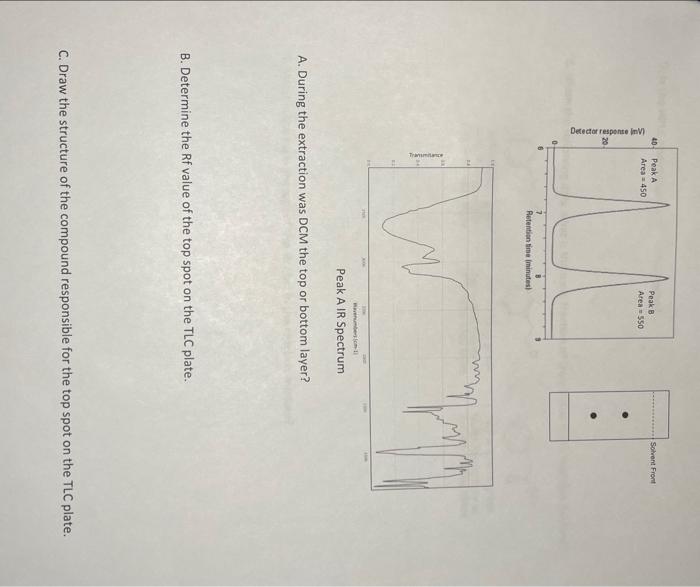

1. A student reacted phenethyl alcohol with PCC (shown below) but wasn't sure what the product of the reaction was going to be. In addition the student only added enough PCC to react with about half of the alcohol. After all the PCC reacted the student carried out an extraction using DCM to isolate the organic compounds present. The student took a TLC and HPLC of the compounds and found that there were two compounds present. The HPLC and TLC are shown below. The student forgot whether he used a reverse phase or normal phase HPLC column. To figure this out he took an IR spectrum of the compound responsible for peak A. A. During the extraction was DCM the top or bottom layer? B. Determine the Rf value of the top spot on the TLC plate. C. Draw the structure of the compound responsible for the top spot on the TLC plate. D. Is the HPLC reverse phase or normal phase? E. Given the following data what is the mole fraction of the alcohol in the reaction mixture? MW=122.17g/molMolarAbsorptivityat270nm=4500M/cmMW=120.15g/molMolarAbsorptivityat270nm=3100M/cmMW=136.15g/molMolarAbsorptivityat270nm=8505M/cm F. What is the mass percent of the alcohol in the reaction mixture? 1. A student reacted phenethyl alcohol with PCC (shown below) but wasn't sure what the product of the reaction was going to be. In addition the student only added enough PCC to react with about half of the alcohol. After all the PCC reacted the student carried out an extraction using DCM to isolate the organic compounds present. The student took a TLC and HPLC of the compounds and found that there were two compounds present. The HPLC and TLC are shown below. The student forgot whether he used a reverse phase or normal phase HPLC column. To figure this out he took an IR spectrum of the compound responsible for peak A. A. During the extraction was DCM the top or bottom layer? B. Determine the Rf value of the top spot on the TLC plate. C. Draw the structure of the compound responsible for the top spot on the TLC plate. D. Is the HPLC reverse phase or normal phase? E. Given the following data what is the mole fraction of the alcohol in the reaction mixture? MW=122.17g/molMolarAbsorptivityat270nm=4500M/cmMW=120.15g/molMolarAbsorptivityat270nm=3100M/cmMW=136.15g/molMolarAbsorptivityat270nm=8505M/cm F. What is the mass percent of the alcohol in the reaction mixture 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


