Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer with calculations. Thank you! A high-temperature reservoir at T=300C is used as a heat source for a heat engine at steady state with
Please answer with calculations. Thank you!
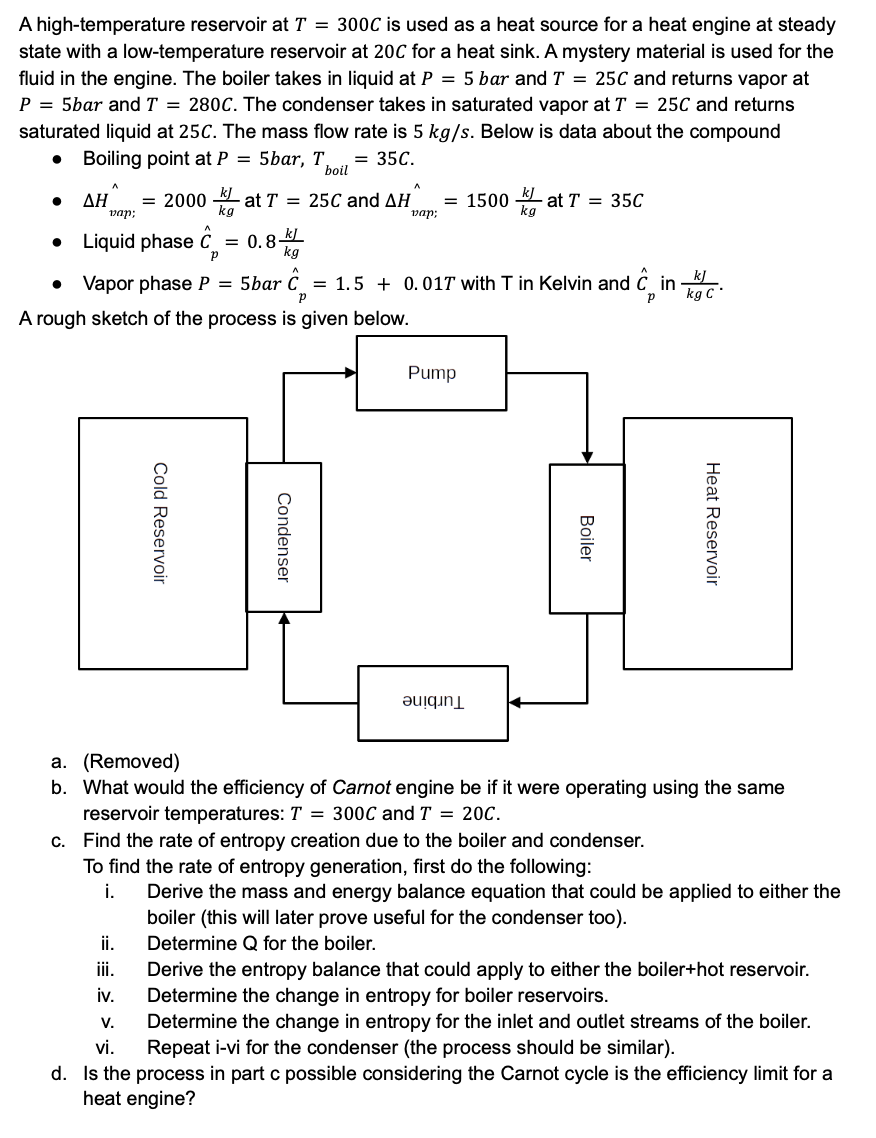
A high-temperature reservoir at T=300C is used as a heat source for a heat engine at steady state with a low-temperature reservoir at 20C for a heat sink. A mystery material is used for the fluid in the engine. The boiler takes in liquid at P=5bar and T=25C and returns vapor at P=5bar and T=280C. The condenser takes in saturated vapor at T=25C and returns saturated liquid at 25C. The mass flow rate is 5kg/s. Below is data about the compound - Boiling point at P=5bar,Tboil=35C. - H^vap;n^=2000kgkJ at T=25C and H^vap;=1500kgkJ at T=35C - Liquid phase C^p=0.8kgkJ - Vapor phase P=5 bar C^p=1.5+0.01T with T in Kelvin and C^p in kgCkJ. A rough sketch of the process is given below. a. (Removed) b. What would the efficiency of Carnot engine be if it were operating using the same reservoir temperatures: T=300C and T=20C. c. Find the rate of entropy creation due to the boiler and condenser. To find the rate of entropy generation, first do the following: i. Derive the mass and energy balance equation that could be applied to either the boiler (this will later prove useful for the condenser too). ii. Determine Q for the boiler. iii. Derive the entropy balance that could apply to either the boiler+hot reservoir. iv. Determine the change in entropy for boiler reservoirs. v. Determine the change in entropy for the inlet and outlet streams of the boiler. vi. Repeat i-vi for the condenser (the process should be similar). d. Is the process in part c possible considering the Carnot cycle is the efficiency limit for a heat engineStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


