Answered step by step
Verified Expert Solution
Question
1 Approved Answer
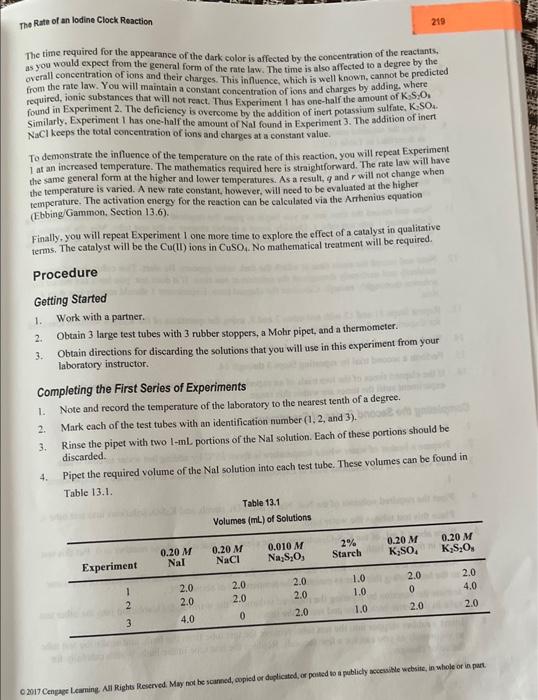
please anwer 2 a 218 Experiment 1: Rate =N2SO22O2I) Experiment 2: Rate =(2(S,Oii])[1]=2 ratel The rate in the second experiment will be 2 t times


please anwer 2 a
218 Experiment 1: Rate =N2SO22O2I) Experiment 2: Rate =(2(S,Oii])[1]=2 ratel The rate in the second experiment will be 2 t times the original rate. In Experiment 3, the initial concentration of S2O2 - ions will be the same as that in Experiasent. 1, but the concentration of I ions will be doubled. The rate law will be Experiment 3: Rate =k[S:O2]Z(2[T])f=2 rate The nate in this experiment will be 2 times the original rate. You can determine the trae values of q and r from the ratios rate/rate, and rate / /ratel. After you have evaluated the exponents, yoa will know the general form of the rate law, but the value of the rate constant will be unknown. You can obtain it from the rate law and any one of the three rates. Each rate should yield the same rate constant within experimental error. The rate of the reaction is given by. therefore you will need to know or measure [S2O32], the initial change in the concentration of S2O22 ions, and At, the time elapsed during that change, as well as the initial concentrations of [S2O82] and 1 ions. An easy way to obtain A[S2O52] is by coupling another reaction to the one we are studying. The new reaction is the reduction of I2b2Na2S2O2 (sodium thiosal fate). The net ionic equation for this reaction is Where Su62 is the tetrathionate ion. It is important ro remember that this reaction is used only as a means of stidying the rate of the first reaction. The new reaction is fast. As a result, l2 is consumed in this reaction as fast as it is formed in the first reaction. Essentially, there can be no l2 present as long as there are S2O32 ions present. However, these ions are being consumed because of the second reaction. As soon as all the S2O32 ions have reacted, 12 from the first reaction begins to accumulate in the solution. You can detect the sudden presence of I. by the sudden appearance of the dark color from the interaction of I2 with starch, which will be used as an indicator. You will know the change in the amount of the persulfate ion, A2S2O222. at the instant that this dark color appears. How? As you will show in the Prelaboratory Assignment, the stoichiometry and relative rates of the coupled reactions require the following relationship between N[S2O52] and [S2O32] : [S2O32]=(B)[S2O32] where [S2O32]=initial[S2O32]-final[S2O33] Essentially all the S2O32 ions will have reacted when the dark color appears, so the final concentration of these ions is essentially zero, and A[S2O32] becomes A[S2O32]=initial[S2O32] If we keep the initial concentration of S2O32 ions at a small value the change in thiosulfate, [S2O32]2], will be small and [S2O22] will be even smaller. As a consequence, there will be little change in the concentration of the reactants during the elapsed time t. This is a necessary condition for the initialrates method. o 2017 Cengage Learnite. All Righs Reaened. May mot he veanned, copied or duplicated, er posted to a publicly accestible website, in whole or iat The Rate of an lodine Clock Reaction The time required for the appearance of the dark color is affected by the concentration of the reactants. as you would expect from the general form of the rate law. The time is also affected to a degree by the overall concentration of ions and their charges. This influence, which is well known, cannot be predicted from the rate law. You will maintain a constant concentration of ions and charges by adding. where required, ionic substances that will not react. Thus Experiment t has one-half the amount of K2S2O3 found in Experiment 2. The deficiency is overcome by the addition of inert potassium sulfate, K,SO4. Similarly. Experiment 1 has one-half the amount of Nal found is Experiment 3. The addition of inert NaCl keeps the total concentration of ions and eharges at a constant value. To demonstrate the influence of the temperature on the rate of this reaction. you will repeat Experiment I at an increased temperature. The mathematics required here is straightforward. The rate law will have the same general form at the higher and lower tempenatures. As a result, q and r will not change when the temperature is varied. A new rate constant. however, will need to be evaluated at the higher temperature. The activation energy for the reaction can be calculated via the Arhenius equation (Ebbing/Gammon, Section 13.6). Finally, you will repeat Experiment 1 one more time to explore the effect of a catalyst in qualitative terms. The catalyst will be the Cu(II) ions in CuSO4. No mathematical treatment will be required. Procedure Getting Started 1. Work with a partner. 2. Obtain 3 large test tubes with 3 rubber stoppers, a Mohr pipet, and a thermometer. 3. Obtain directions for disearding the solutions that you will use in this experiment from your laboratory instructor. Completing the First Series of Experiments 1. Note and record the temperature of the laboratory to the nearest tenth of a degree. 2. Mark each of the test tubes with an identification number (1,2, and 3). 3. Rinse the pipet with two L-mL portions of the Nal solution. Each of these portions should be discarded. 4. Pipet the required volume of the Nal solution into each test tube. These volumes can be found in Tnble 13.1. 2. a. Write the balancedchemical equation for the reaction whose rate is being studied. S2O82+2I2SO42+I2 b. Write the balanced chemical equation for the reaction that will enable you to know [S K2S2O8+2NaII2+K2SO4+Na2SO4 c. Why are these coupled reactions called an iodine clock reaction? Because like a clock you can set the da color by altering the concentration. Also, the pears so abruptly, that its compared to the The dark color comes from the interact of iodine (I2) and starch Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


