Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please as soon as possible thanks 0. The charge of the colloidal particles of the AgCl sol is negative. Use the Hardy-Schultze rule and for
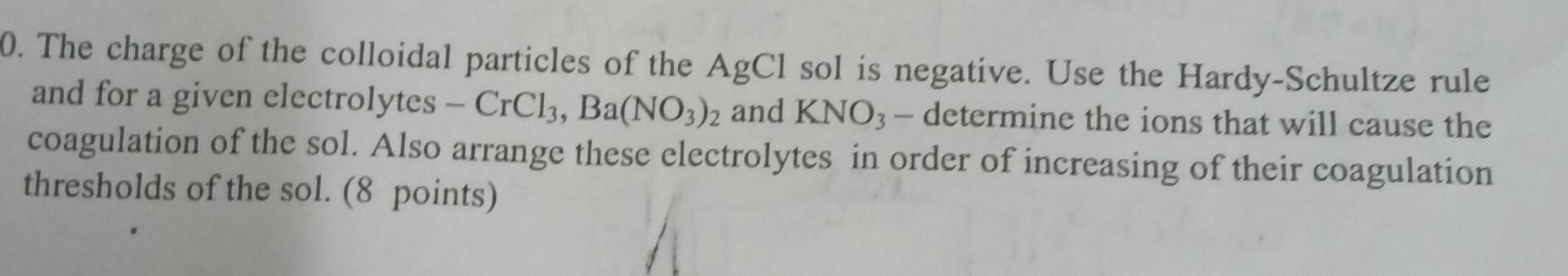
please as soon as possible thanks
0. The charge of the colloidal particles of the AgCl sol is negative. Use the Hardy-Schultze rule and for a given electrolytes - CrCl3, Ba(NO3)2 and KNO3 - determine the ions that will cause the coagulation of the sol. Also arrange these electrolytes in order of increasing of their coagulation thresholds of the sol. (8 points)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


