Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please asap (a) The wavelength of a photon required to cause the dissociation of a molecule into its constituent atoms, is 270nm. Given the information
please asap 
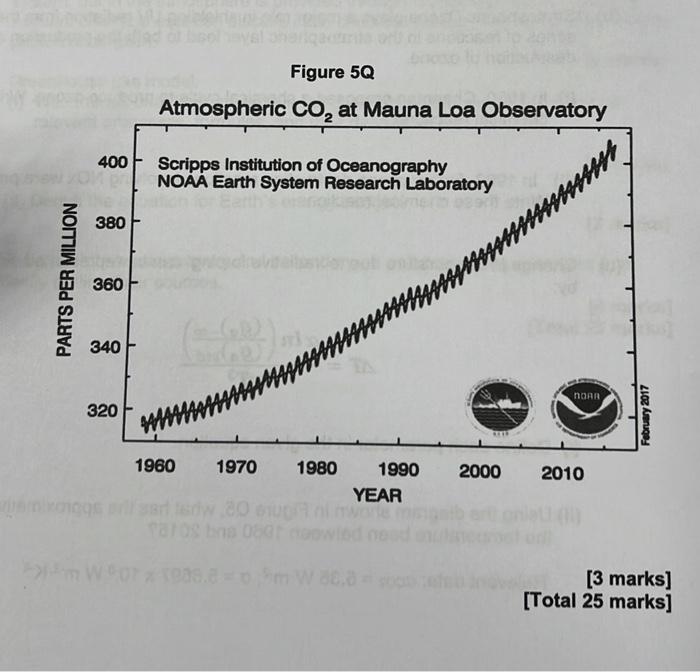
(a) The wavelength of a photon required to cause the dissociation of a molecule into its constituent atoms, is 270nm. Given the information below, calculate the corresponding bond dissociation energy of the molecule, in kJmol1. Relevant information: h=6.6261034Js;c=3108ms1; Avogadro's constant =6.0221023mol1 [6 marks] (b) Stratospheric Ozone plays a major role in shielding UV radiation from the Sun. A series of reactions in the stratospheric layer lead to both the production and destruction of ozone. (i) In 1930, Chapman proposed a chemical cycle to produce ozone. Write these chemical reactions. [3 marks] (ii) In 1960, further ozone destruction reactions involving NOx were proposed. Write these chemical reactions. [7 marks] (c) Change in temperature due to 'radiative forcing' can be represented simply by: T=4T3ln((ga)std(ga)) (i) Define each parameter in the given equation. [6 marks] (ii) Using the diagram shown in Figure Q5, what has the approximative change in the temperature been between 1960 and 2015 ? Relevant data: c02=5.35Wm2;=5.6697108Wm2K4 [3 marks] [Total 25 marks] 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


