Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please assist with selectivity and conversion calculations for the acrylic acid production process from propylene. Flow sheet consist of a reactor, seperation tower and distillation
Please assist with selectivity and conversion calculations for the acrylic acid production process from propylene.
Flow sheet consist of a reactor, seperation tower and distillation tower.
Include mass balance with stoichiometry of reactions as it takes place in the reactor.
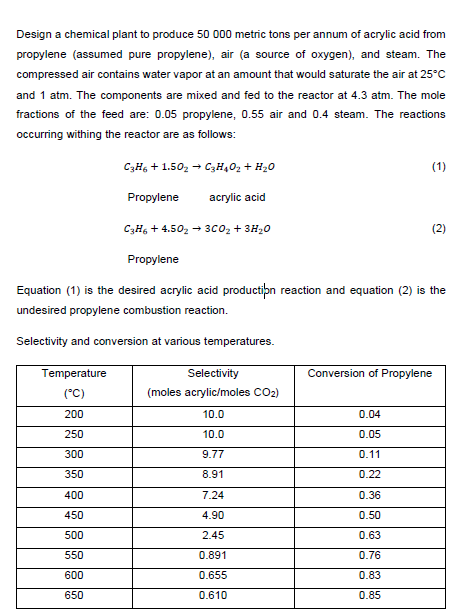
Design a chemical plant to produce 50000 metric tons per annum of acrylic acid from propylene (assumed pure propylene), air (a source of oxygen), and steam. The compressed air contains water vapor at an amount that would saturate the air at 25C and 1atm. The components are mixed and fed to the reactor at 4.3atm. The mole fractions of the feed are: 0.05 propylene, 0.55 air and 0.4 steam. The reactions occurring withing the reactor are as follows: C3H6+1.5O2C3H4O2+H2O Propylene acrylic acid C3H6+4.5O23CO2+3H2O Propylene Equation (1) is the desired acrylic acid production reaction and equation (2) is the undesired propylene combustion reaction. Selectivity and conversion at various temperaturesStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


