Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please calculate all values required Consider the initial composition given by 2CO+2O2 (on mole basis), which is subject to the dry oxidation reaction (CO+0.5O2CO2), which
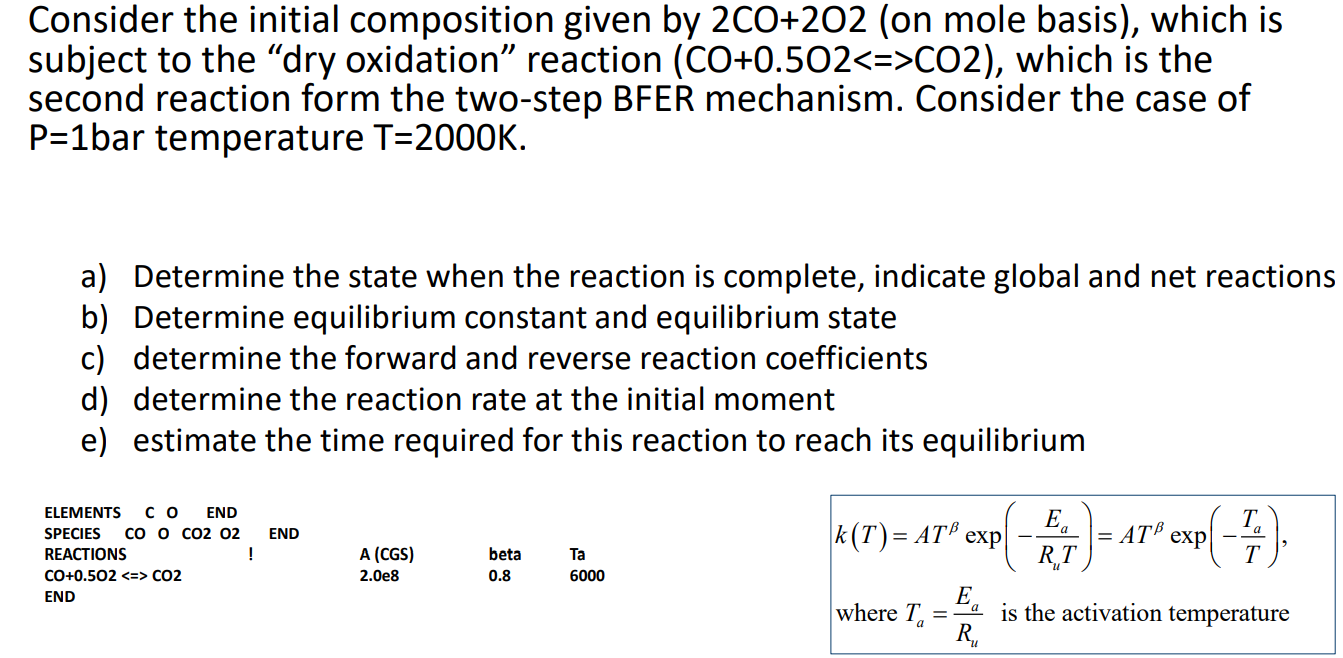
please calculate all values required
Consider the initial composition given by 2CO+2O2 (on mole basis), which is subject to the "dry oxidation" reaction (CO+0.5O2CO2), which is the second reaction form the two-step BFER mechanism. Consider the case of P=1 bar temperature T=2000K. a) Determine the state when the reaction is complete, indicate global and net reactions b) Determine equilibrium constant and equilibrium state c) determine the forward and reverse reaction coefficients d) determine the reaction rate at the initial moment e) estimate the time required for this reaction to reach its equilibrium k(T)=ATexp(RuTEa)=ATexp(TTa), where Ta=RuEa is the activation temperature
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


