Choose from the conversion factors given to complete the following diagram of amount-mass-number relationships for a compound. molar mass (g/mol) of compound atomic mass
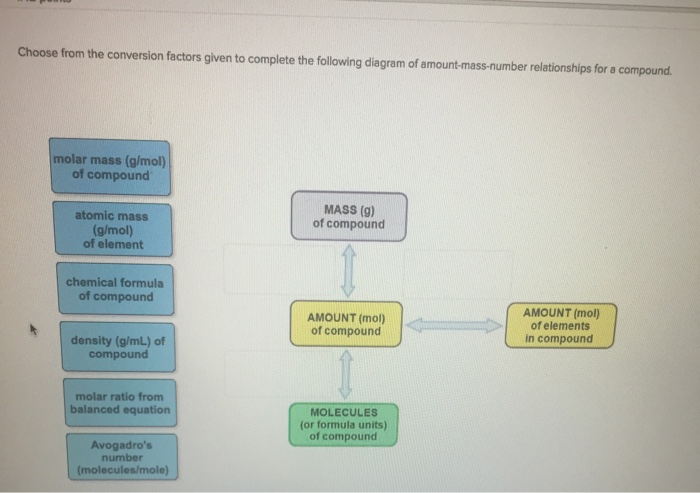
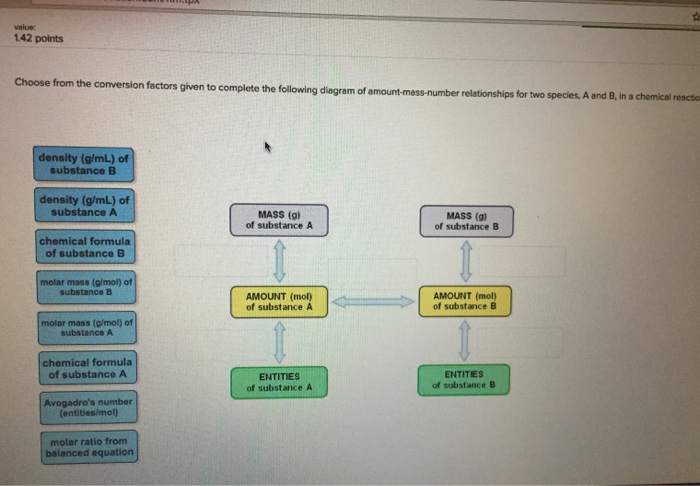
Choose from the conversion factors given to complete the following diagram of amount-mass-number relationships for a compound. molar mass (g/mol) of compound atomic mass (g/mol) of element chemical formula of compound density (g/mL) of compound molar ratio from balanced equation Avogadro's number (molecules/mole) MASS (g) of compound AMOUNT (mol) of compound MOLECULES (or formula units) of compound AMOUNT (mol) of elements in compound value: 142 points Choose from the conversion factors given to complete the following diagram of amount-mass-number relationships for two species, A and B, in a chemical reaction density (g/mL) of substance B density (g/mL) of substance A chemical formula of substance B molar mass (g/mol) of substance B molar mass (g/mol) of substance A chemical formula of substance A Avogadro's number. (entities/mol) molar ratio from balanced equation MASS (g) of substance A AMOUNT (mol) of substance A ENTITIES of substance A MASS (g) of substance B AMOUNT (mol) of substance B ENTITIES of substance B
Step by Step Solution
3.37 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


