Question: -PLEASE COME UP WITH MADE UP REALISTIC DATA. THERE ISNT NO DATA SO MAKE YOUR OWN. -PLEASE COMPLETE THE TABLE BELOW! THANKS QUESTION: Surface Tension:

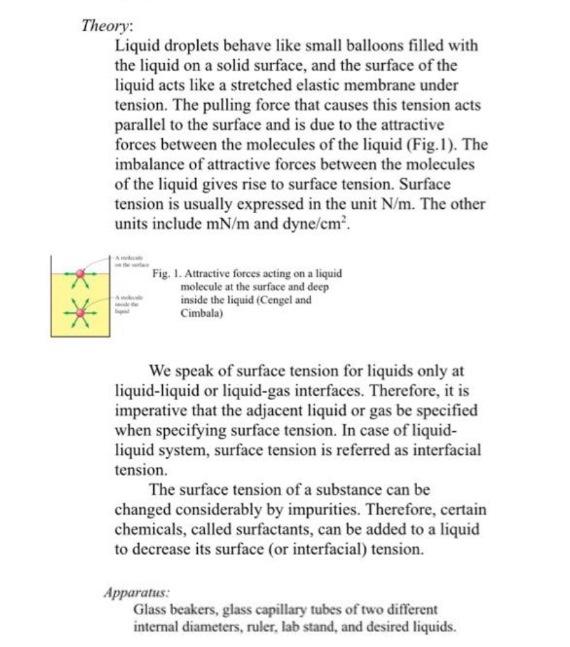


-PLEASE COME UP WITH MADE UP REALISTIC DATA. THERE ISNT NO DATA SO MAKE YOUR OWN. -PLEASE COMPLETE THE TABLE BELOW! THANKS QUESTION: Surface Tension: Fluid Capillary radius (mm) Capillary rise (cm) Water Corn syrup PLEASE READ DOCUMENT BELOW ON HOW TO SOLVE FOR THE BOX ABOVE. THANKS 2. Surface Tension: Objective: To measure the surface tension of distilled water and corn syrup Theory: Liquid droplets behave like small balloons filled with the liquid on a solid surface, and the surface of the liquid acts like a stretched elastic membrane under tension. The pulling force that causes this tension acts parallel to the surface and is due to the attractive forces between the molecules of the liquid (Fig.1). The imbalance of attractive forces between the molecules of the liquid gives rise to surface tension. Surface tension is usually expressed in the unit N/m. The other units include mN/m and dyne/cm. Fig. 1. Attractive forces acting on a liquid molecule at the surface and deep inside the liquid (Cengel and Cimbala) We speak of surface tension for liquids only at liquid-liquid or liquid-gas interfaces. Therefore, it is imperative that the adjacent liquid or gas be specified when specifying surface tension. In case of liquid- liquid system, surface tension is referred as interfacial tension. The surface tension of a substance can be changed considerably by impurities. Therefore, certain chemicals, called surfactants, can be added to a liquid to decrease its surface (or interfacial) tension. Apparatus: Glass beakers, glass capillary tubes of two different internal diameters, ruler, lab stand, and desired liquids. Method: An interesting consequence of surface tension is capillary effect, which is the rise or fall of a liquid in a small-diameter tube inserted into the liquid. Such narrow tubes or confined flow channels are called capillaries. The curved free surface of a liquid in a capillary tube is called the meniscus. It is commonly observed that water in a glass contained curves up slightly at the edges where it touches the glass surface; but the opposite occurs for mercury: it curves down at the edges (Fig. 2). This can be used to quantify the strength of the capillary effect in terms of a quantity called contact (or wetting) angle. Contact angle is defined as the angle that the tangent to the liquid surface makes with the solid surface at the point of contact. Fig. 2. The contact angle for wetting and nonwetting fluids (Cengel and Cimbala) The magnitude of the capillary rise in a circular tube can be used to measure the surface tension for liquid-gas (or interfacial tension for liquid-liquid) system. This method of measuring surface tension is called "capillary rise method" (Fig. 3). el.com Fig. 3. The forces acting on a liquid column that has risen in a tube due to the capillary effect (Cengel and Cimbala) rhe. - P.) 2004 A force balance for the system shown in Fig 3 correlates the capillary rise, h, to surface tension. Where is the surface tension (N/m) between liquid and gas phase, r is the internal radius of capillary tube (m), h is height of capillary rise (m), P. and p, are the densities (kg/m) of liquid and gas phase respectively, cose (which is assumed to be 0 for air-water system) is the equilibrium contact angle in degrees and g is acceleration due to gravity (9.81 m/s) The above equation is also valid for nonwetting liquids (such as mercury in glass) and gives the capillary drop. Method: Insert a glass capillary tube of given internal diameter in a beaker filled with the liquid for which you want to measure the surface tension. Use lab stand and clamp to hold capillary tube in the liquid. Record the capillary rise height using a ruler. Repeat the same for another diameter glass capillary tube. Conduct two or three trials for each liquid. Analysis: Compare with reference values. Make sure to cite your source(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


