 Please complete A through C
Please complete A through C
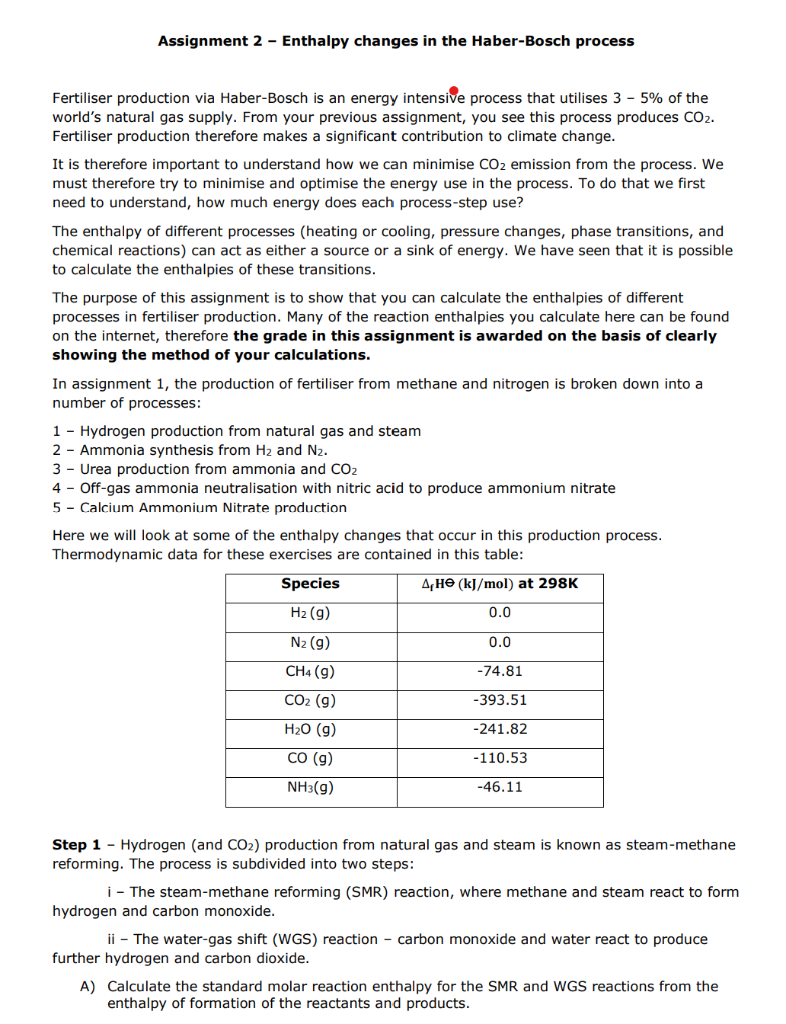
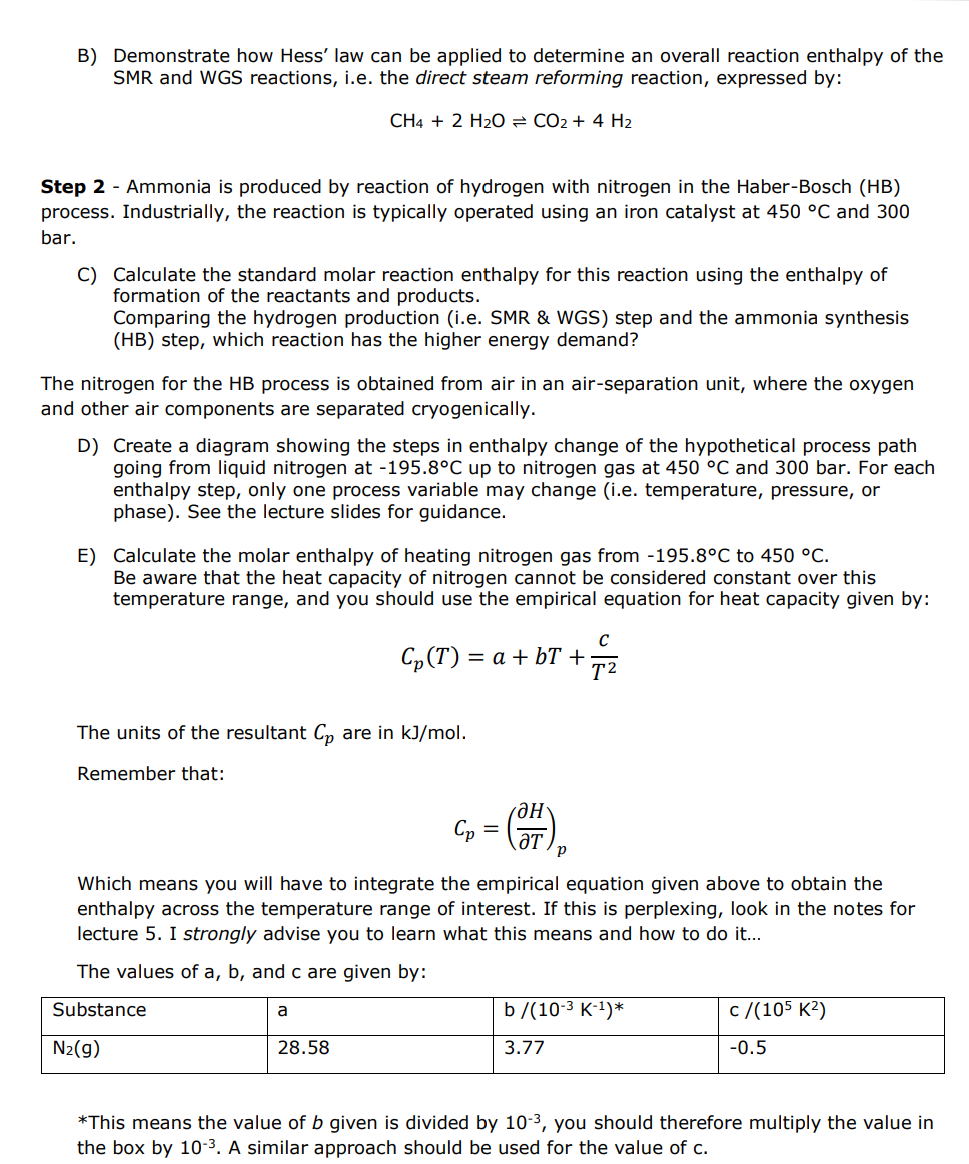
Assignment 2 - Enthalpy changes in the Haber-Bosch process Fertiliser production via Haber-Bosch is an energy intensive process that utilises 35% of the world's natural gas supply. From your previous assignment, you see this process produces CO2. Fertiliser production therefore makes a significant contribution to climate change. It is therefore important to understand how we can minimise CO2 emission from the process. We must therefore try to minimise and optimise the energy use in the process. To do that we first need to understand, how much energy does each process-step use? The enthalpy of different processes (heating or cooling, pressure changes, phase transitions, and chemical reactions) can act as either a source or a sink of energy. We have seen that it is possible to calculate the enthalpies of these transitions. The purpose of this assignment is to show that you can calculate the enthalpies of different processes in fertiliser production. Many of the reaction enthalpies you calculate here can be found on the internet, therefore the grade in this assignment is awarded on the basis of clearly showing the method of your calculations. In assignment 1 , the production of fertiliser from methane and nitrogen is broken down into a number of processes: 1 - Hydrogen production from natural gas and steam 2 - Ammonia synthesis from H2 and N2. 3 - Urea production from ammonia and CO2 4 - Off-gas ammonia neutralisation with nitric acid to produce ammonium nitrate 5 - Calcium Ammonium Nitrate production Here we will look at some of the enthalpy changes that occur in this production process. Thermodynamic data for these exercises are contained in this table: Step 1 - Hydrogen (and CO2 ) production from natural gas and steam is known as steam-methane reforming. The process is subdivided into two steps: i - The steam-methane reforming (SMR) reaction, where methane and steam react to form hydrogen and carbon monoxide. ii - The water-gas shift (WGS) reaction - carbon monoxide and water react to produce further hydrogen and carbon dioxide. A) Calculate the standard molar reaction enthalpy for the SMR and WGS reactions from the enthalpy of formation of the reactants and products. B) Demonstrate how Hess' law can be applied to determine an overall reaction enthalpy of the SMR and WGS reactions, i.e. the direct steam reforming reaction, expressed by: CH4+2H2OCO2+4H2 Step 2 - Ammonia is produced by reaction of hydrogen with nitrogen in the Haber-Bosch (HB) process. Industrially, the reaction is typically operated using an iron catalyst at 450C and 300 bar. C) Calculate the standard molar reaction enthalpy for this reaction using the enthalpy of formation of the reactants and products. Comparing the hydrogen production (i.e. SMR \& WGS) step and the ammonia synthesis (HB) step, which reaction has the higher energy demand? The nitrogen for the HB process is obtained from air in an air-separation unit, where the oxygen and other air components are separated cryogenically. D) Create a diagram showing the steps in enthalpy change of the hypothetical process path going from liquid nitrogen at 195.8C up to nitrogen gas at 450C and 300 bar. For each enthalpy step, only one process variable may change (i.e. temperature, pressure, or phase). See the lecture slides for guidance. E) Calculate the molar enthalpy of heating nitrogen gas from 195.8C to 450C. Be aware that the heat capacity of nitrogen cannot be considered constant over this temperature range, and you should use the empirical equation for heat capacity given by: Cp(T)=a+bT+T2c The units of the resultant Cp are in kJ/mol. Remember that: Cp=(TH)p Which means you will have to integrate the empirical equation given above to obtain the enthalpy across the temperature range of interest. If this is perplexing, look in the notes for lecture 5. I strongly advise you to learn what this means and how to do it... The values of a,b, and c are given by: *This means the value of b given is divided by 103, you should therefore multiply the value in the box by 103. A similar approach should be used for the value of c

 Please complete A through C
Please complete A through C





