Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please complete all questions 1. (a) Write balanced molecular equations for the following potential precipitation reactions. Indicate the states of reactants and products [(aq) or
please complete all questions 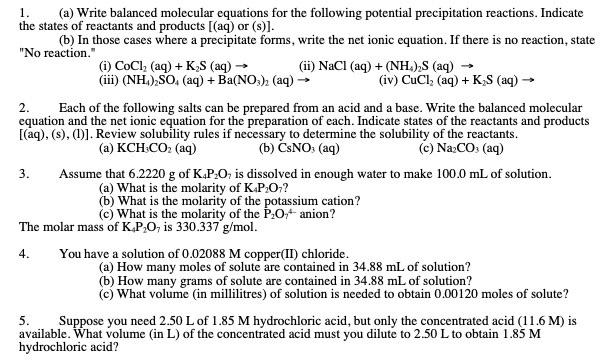
1. (a) Write balanced molecular equations for the following potential precipitation reactions. Indicate the states of reactants and products [(aq) or (s)]. (b) In those cases where a precipitate forms, write the net ionic equation. If there is no reaction, state "No reaction." 2. Each of the following salts can be prepared from an acid and a base. Write the balanced molecular equation and the net ionic equation for the preparation of each. Indicate states of the reactants and products [(aq),(s),(1)]. Review solubility rules if necessary to determine the solubility of the reactants. (a) KCH3CO2 (aq) (b) CsNO3(aq) (c) Na2CO3 (aq) 3. Assume that 6.2220g of K4P2O7 is dissolved in enough water to make 100.0mL of solution. (a) What is the molarity of K4P2O7 ? (b) What is the molarity of the potassium cation? (c) What is the molarity of the P2O74 anion? The molar mass of K4P2O7 is 330.337g/mol. 4. You have a solution of 0.02088M copper(II) chloride. (a) How many moles of solute are contained in 34.88mL of solution? (b) How many grams of solute are contained in 34.88mL of solution? (c) What volume (in millilitres) of solution is needed to obtain 0.00120 moles of solute? 5. Suppose you need 2.50L of 1.85M hydrochloric acid, but only the concentrated acid (11.6M) is available. What volume (in L) of the concentrated acid must you dilute to 2.50L to obtain 1.85M hydrochloric acid 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


