Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please detail matematicaly A certain gas has the following equations of state, where B is a positive constant The system temperature Ti and initial pressure
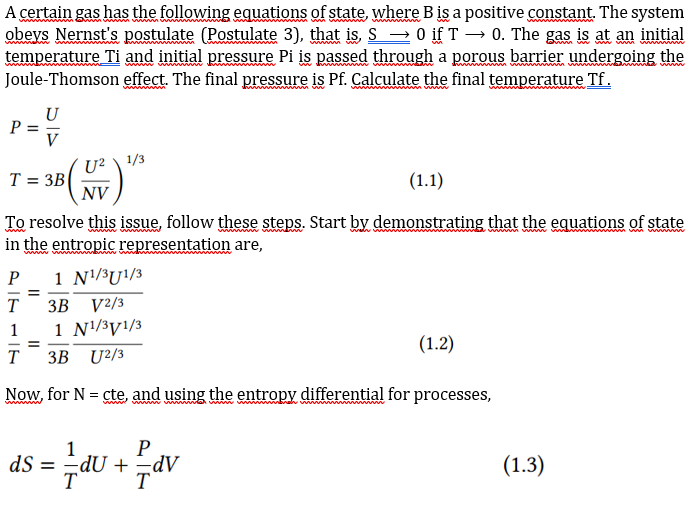

Please detail matematicaly
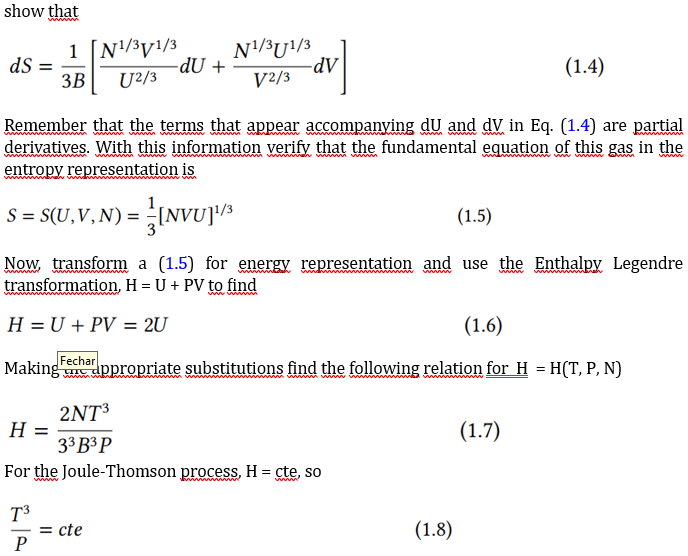
A certain gas has the following equations of state, where B is a positive constant The system temperature Ti and initial pressure Pi is passed through a porous barrier undergoing the Joule-Thomson effect. The final pressure is Pf. Calculate the final temperature Tf. PT=VU=3B(NVU2)1/3 To resolve this issue, follow these steps. Start by demonstrating that the equations of state in the entropic representation are, TP=3B1V2/3N1/3U1/3T1=3B1U2/3N1/3V1/3 Now, for N = cte, and using the entropy differential for processes, dS=T1dU+TPdV show that dS=3B1[U2/3N1/3V1/3dU+V2/3N1/3U1/3dV] Remember that the terms that appear accompanying dU and dV in Eq. (1.4) are partial derivatives. With this information verify that the fundamental equation of this gas in the entropy representation is S=S(U,V,N)=31[NVU]1/3 Now, transform a (1.5) for energy representation and use the Enthalpy Legendre transformation, H=U+PV to find H=U+PV=2U H=33B3P2NT3 For the Joule-Thomson process, H= cte, so PT3=cte To finally find the relationship between pressure and temperature before and after the Joule-Thomson process Tf=Ti(PiPf)1/3 This exercise shows how to determine the state of fluids in a JT process using the tools of thermodynamic theory. The "art" lies in choosing the most appropriate representationStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


