Answered step by step
Verified Expert Solution
Question
1 Approved Answer
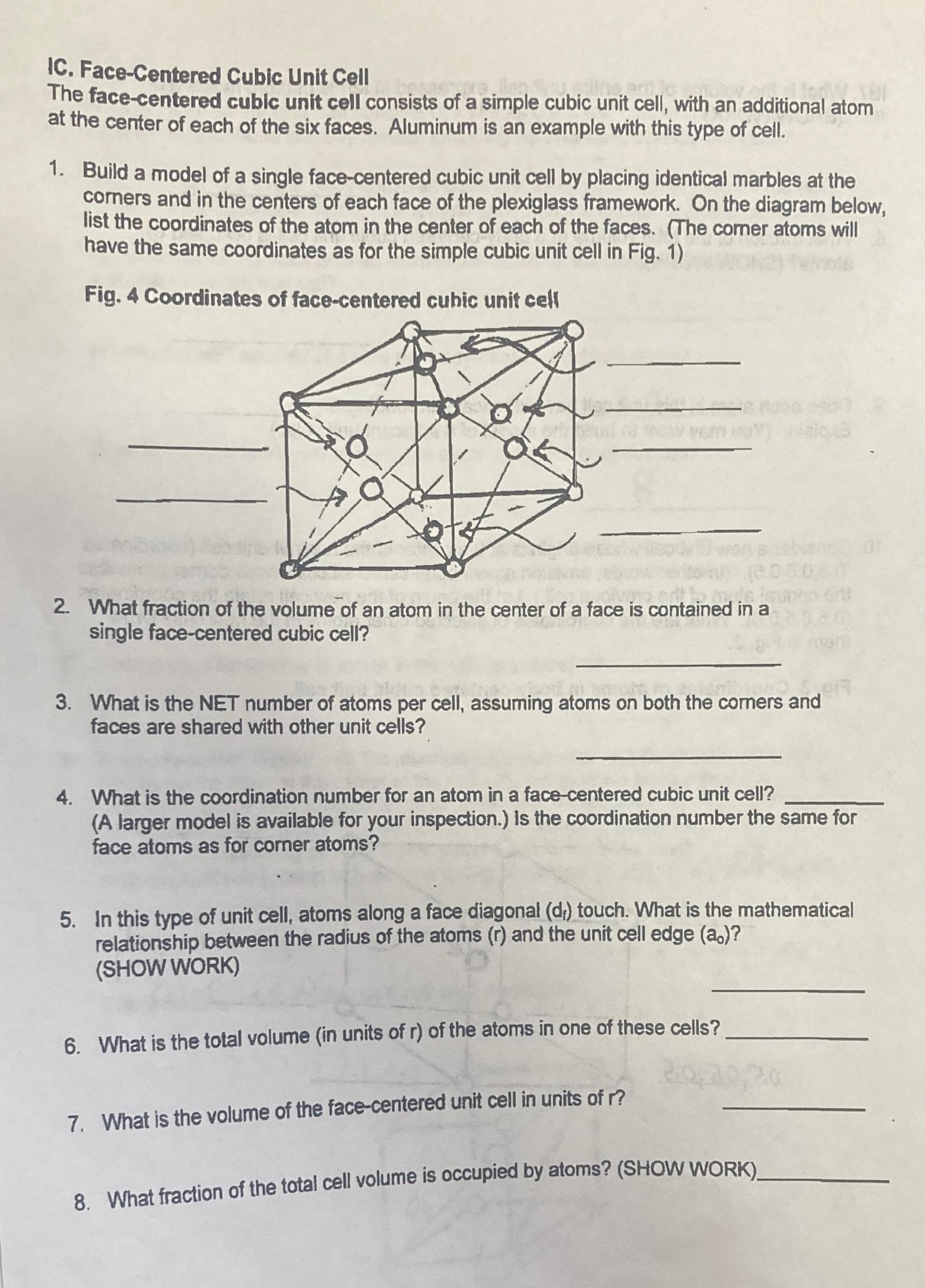
Please do all 8 questions se do all 8 questions C . Face - Centered Cubic Unit Cell The face - centered cublc unit cell
Please do all questions se do all questions
C FaceCentered Cubic Unit Cell
The facecentered cublc unit cell consists of a simple cubic unit cell, with an additional atom at the center of each of the six faces. Aluminum is an example with this type of cell.
Build a model of a single facecentered cubic unit cell by placing identical marbles at the corners and in the centers of each face of the plexiglass framework. On the diagram below, list the coordinates of the atom in the center of each of the faces. The comer atoms will have the same coordinates as for the simple cubic unit cell in Fig.
Fig. Coordinates of facecentered cubic unit cell
What fraction of the volume of an atom in the center of a face is contained in a single facecentered cubic cell?
What is the NET number of atoms per cell, assuming atoms on both the comers and faces are shared with other unit cells?
What is the coordination number for an atom in a facecentered cubic unit cell?
A larger model is available for your inspection. is the coordination number the same tor face atoms as for corner atoms?
In this type of unit cell, atoms along a face diagonal touch. What is the mathematical relationship between the radius of the atoms and the unit cell edge
SHOW WORK
What is the total volume in units of of the atoms in one of these cells
What is the volume of the facecentered unit cell in units of
What fraction of the total cell volume is occupied by atoms? SHOW WORK
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


