Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please don't copy and paste the answer from another chegg post The following data are obtained at 504C for decomposition of dimethyl ether in a
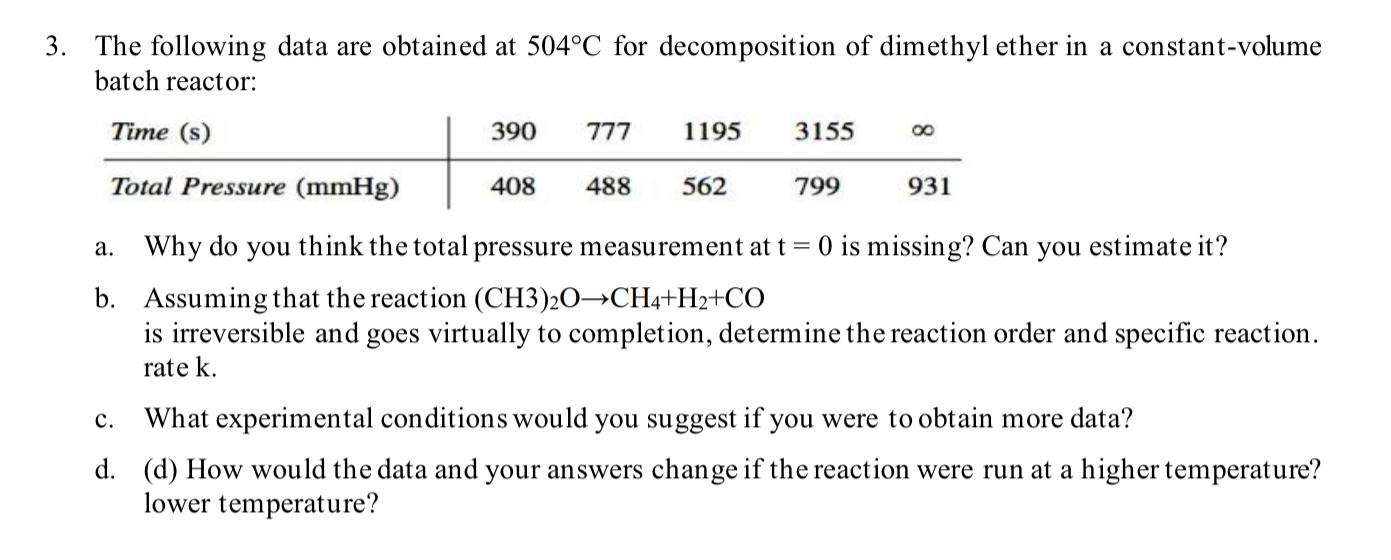
Please don't copy and paste the answer from another chegg post
The following data are obtained at 504C for decomposition of dimethyl ether in a constant-volume batch reactor: a. Why do you think the total pressure measurement at t=0 is missing? Can you estimate it? b. Assuming that the reaction (CH3)2OCH4+H2+CO is irreversible and goes virtually to completion, determine the reaction order and specific reaction. rate k. c. What experimental conditions would you suggest if you were to obtain more data? d. (d) How would the data and your answers change if the reaction were run at a higher temperature? lower temperatureStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


