Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please draw a full reaction mechanism and reaction table! Grignard Reaction with an Ester For the Fall Term course, a Grignard reagent was made by
please draw a full reaction mechanism and reaction table! 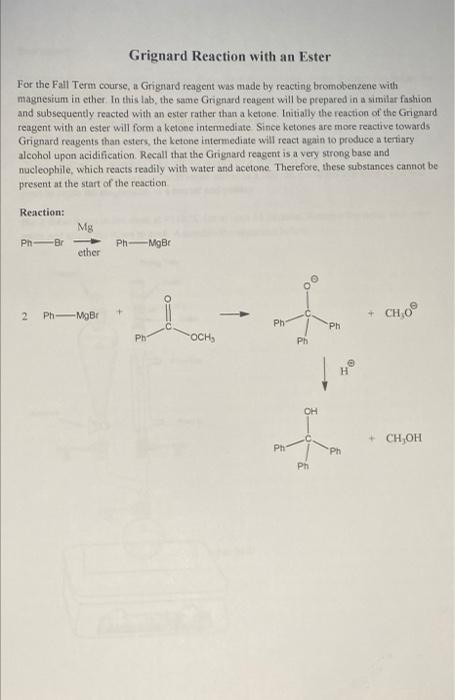


Grignard Reaction with an Ester For the Fall Term course, a Grignard reagent was made by reacting bromobenzene with magnesium in ether. In this lab, the same Grignard reagent will be prepared in a similar fashion and subsequently reacted with an ester rather than a ketone. Initially the reaction of the Grignard reagent with an ester will form a ketone intermediate Since ketones are more reactive towards Grignard reagents than esters, the ketone intermediate will react again to produce a tertiary alcohol upon acidification Recall that the Grignard reagent is a very strong base and nucleophile, which reacts readily with water and acetone. Therefore, these substances cannot be present at the start of the reaction Reaction: Mg Ph-Br Ph-NgBr ether + 2 P MOBI + CHO Ph Ph Ph OCH, Ph H OH t + CH OH Ph Ph Ph Assemble the apparatus except for the 100 mL round bottom flask. Use the condenser without the metal stuff in it to the round bottom flask add 0.7 g of magnesium and a tiny stirbar. Then add 1.0 mL of bromobenzene and 5 mL of anhydrous diethyl ether. Lightly etch the magnesium by pressing with a stir rod at the bottom of the flask. (The flask should on the benchtop of the hood and not be suspended in the airl) Press for about 30 seconds or until you notice a slight cloudiness in the solution Attach the flask to the apparatus above Turn on the water for the condenser. With the stopcock on the separatory funnel in the CLOSED position, add 2 mL of bromobenzene and 10 mL of anhydrous diethyl ether. This portion will be added to the reaction mixture at a later time. Remember that the Grignard reagent is sensitive to water. Therefore, you need to minimize exposure to air of all reagents and solvents. Measure amounts quickly without rushing. Keep solutions in closed containers after taking them out of the reagent bottles and be sure to replace the caps on these bottles. Heat the reaction mixture under a light reflux for 5 minutes using a hot water bath on a hot plate. Note that ether boils at 40 C, so heat should be kept low. Once the reaction actually starts, it should turn cloudy, then eventually a reddish color. You can also check the reaction by removing the heat and see whether or not the solution keeps boiling. If the reaction fails to proceed, see your instructor or teaching assistant Once the reaction does proceed and boils steadily, remove the heat. Have an ice bath in a recrystallizing dish prepared Add the additional solution of 2 ml. of bromobenzene in 10 mL of anhydrous diethyl ether from the separatory funnel dropwise into the reaction mixture with stirring. You will likely need to occasionally remove the stopper from the separatory funnel to keep the flow going. If the solution begins to boil vigorously, stop the addition and add the ice bath. Resume the dropwise addition of the bromobenzene solution Following this addition of the second portion of bromobenzene in diethyl ether, keep the solution boiling at a steady pace for an additional 15 minutes adding a hot water bath if needed. Still have the ice bath ready if needed if the boiling gets too vigorous. Allow the solution to cool to room temperature While the solution is cooling, dissolve 1.7 grams of methyl benzoate in about 10 mL of anhydrous ether and transfer the solution to the separatory funnel. Add the resulting solution dropwise from the separatory funnel to the Grignard reagent solution. The reaction is exothermic and therefore generates its own heat. The degree of boiling should be controlled such that condensation does not travel more than Y up the condenser. After the addition of the methyl benzoate solution is complete, cool the reaction mixture Remove the round bottom flask. Immediately put a greased stopper on the flask and place the flask in a beaker Label the beaker and leave the flask in your hood until the next laboratory period. Pour 15 mL of 6 M sulfuric acid over about 10 grams of crushed ice. Then pour the reaction mixture from the round bottom flask onto this sulfuric acid/ice solution. Use additional ether to aid in the transfer. Stir the resulting mixture with a stirring rod until the solids have dissolved Check the pH of the aqueous layer in the solution. If it is not acidic, add small portions of the 6M sulfuric acid until it is acidic. Add any additional ether to dissolve any solids. Transfer the mixture to a separatory funnel. Shake the funnel while venting frequently, then remove the aqueous layer. Wash the remaining ether layer with 10 mL of 3 M sulfuric (prepared from 6M). followed by two 10 mL portions of saturated sodium bicarbonate (important to vent frequently as CO2 gas is produced upon reaction with excess acid), followed by one 10 mL portion of brine. Dry the ether layer with a layer of calcium chloride covering the bottom of a 50 mL Erlenmeyer flask. Let the solution stand for 5 minutes. Water present will be observed as a cloudy suspension 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


