Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please draw and label thank u model 1 is included. second photo Model 2: Saponification of triacylglycerols Saponification is a method for making soap that
please draw and label
thank u 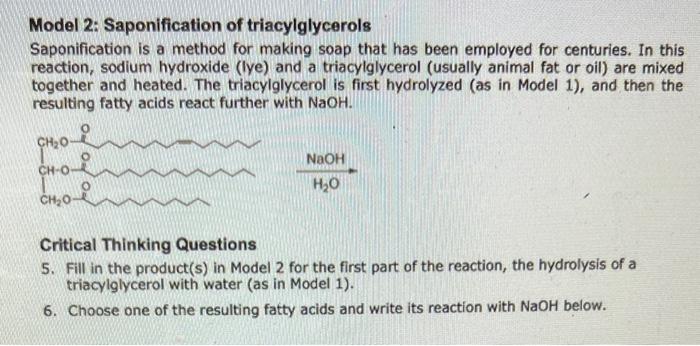

model 1 is included. second photo
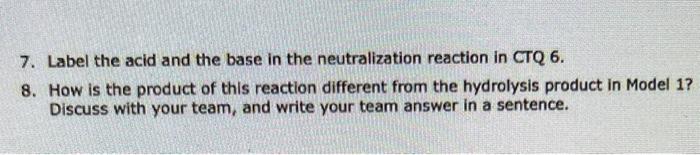
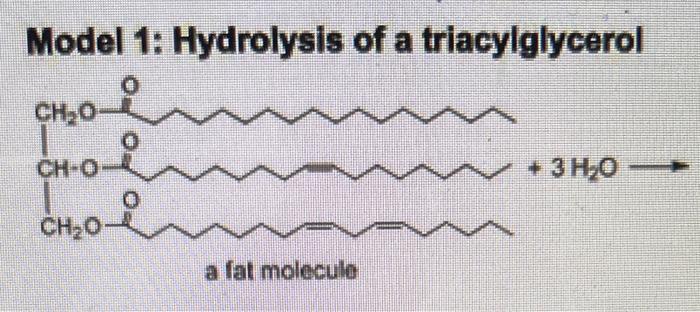
Model 2: Saponification of triacylglycerols Saponification is a method for making soap that has been employed for centuries. In this reaction, sodium hydroxide (Iye) and a triacylglycerol (usually animal fat or oil) are mixed together and heated. The triacylglycerol is first hydrolyzed (as in Model 1), and then the resulting fatty acids react further with NaOH. H2ONaOH Critical Thinking Questions 5. Fill in the product(s) in Model 2 for the first part of the reaction, the hydrolysis of a triacylglycerol with water (as in Model 1). 6. Choose one of the resulting fatty acids and write its reaction with NaOH below. 7. Label the acid and the base in the neutralization reaction in CTQ 6. 8. How is the product of this reaction different from the hydrolysis product in Model 1 ? Discuss with your team, and write your team answer in a sentence. Model 1: Hydrolysis of a triacylglycerol Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


