Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please explain 3ii. i dont understand it at all. please break it down in simple terms. 3. Consider compound A. double bonds are -Spa SP
please explain 3ii. i dont understand it at all. please break it down in simple terms. 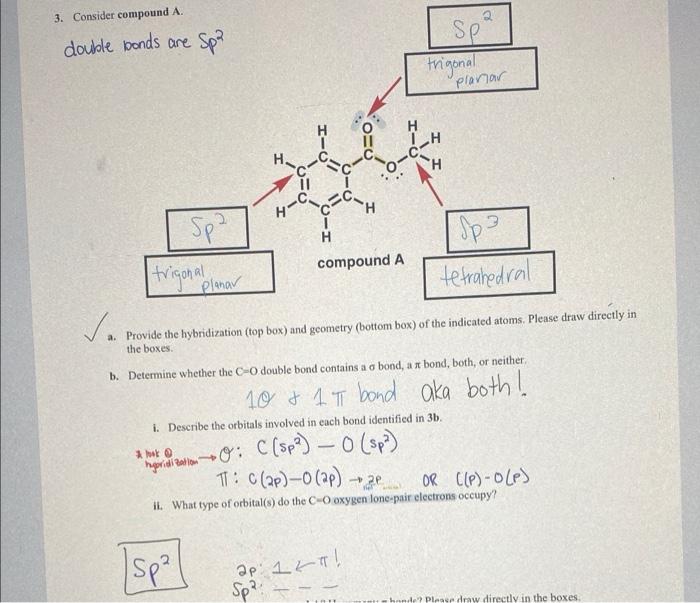
3. Consider compound A. double bonds are -Spa SP trigonal planar H CH CH H C1 11 H-C C Spa compound A I Spa tetrahedral trigonal Planar va a. Provide the hybridization (top box) and geometry (bottom box) of the indicated atoms. Please draw directly in the boxes b. Determine whether the CO double bond contains a o bond, a x bond, both, or neither i. Describe the orbitals involved in each bond identified in 3b. - 10 & 1 T bond aka both! on the comm0: C (Spa) 0 (sp) TT: C(2P) - (ap) OR ((P)-OLP) - 2 il. What type of orbital(s) do the Cooxygen lonc pair electrons occupy? Isp?] ap 1 ka! Sp?! hande? Please draw directly in the boxes 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


