Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please explain both parts of this question. Thank you A gas vessel contains 40.0g of oxygen and 40.0g of helium with a total pressure of
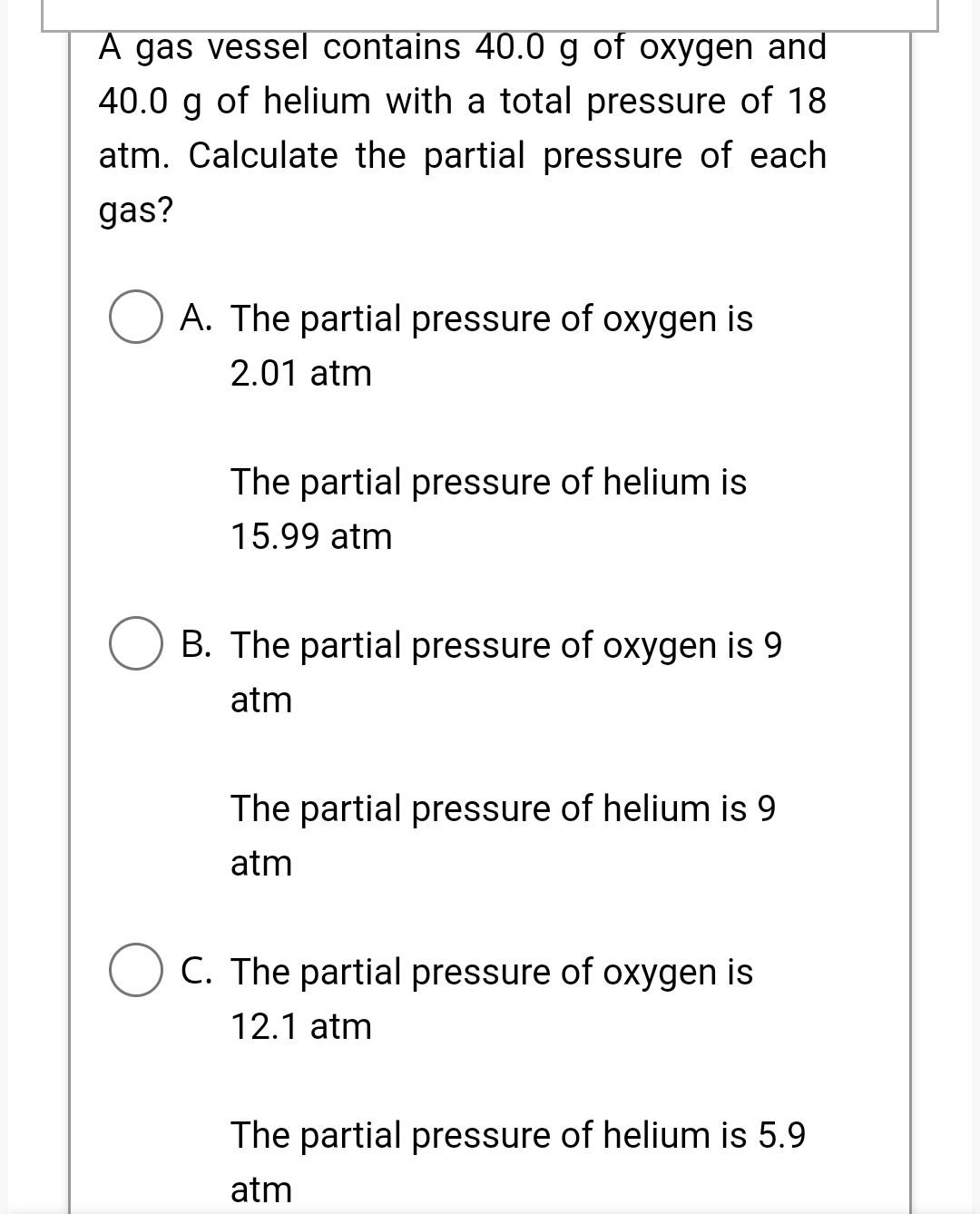


Please explain both parts of this question. Thank you
A gas vessel contains 40.0g of oxygen and 40.0g of helium with a total pressure of 18 atm. Calculate the partial pressure of each gas? A. The partial pressure of oxygen is 2.01 atm The partial pressure of helium is 15.99atm B. The partial pressure of oxygen is 9 atm The partial pressure of helium is 9 atm C. The partial pressure of oxygen is 12.1atm The partial pressure of helium is 5.9 B. The partial pressure of oxygen is 9 atm The partial pressure of helium is 9 atm C. The partial pressure of oxygen is 12.1 atm The partial pressure of helium is 5.9 atm D. None of above The vapor pressure of Chloroform (CHCl3) is 197mmHg at 23.0C; and 448mmHg at 45.0C. Calculate the molar heat of vaporization and the normal boiling point of Chloroform. A. 29.2kJ;344K B. 29.2kJ;234K C. 29.2kJ;334K D. 29.2J;334KStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


