Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please explain clearly 1. Methanol is produced in the reaction of carbon monoxide and hydrogen: CO + 2H2 CH3OH Following Figure illustrates a steady-state process
please explain clearly 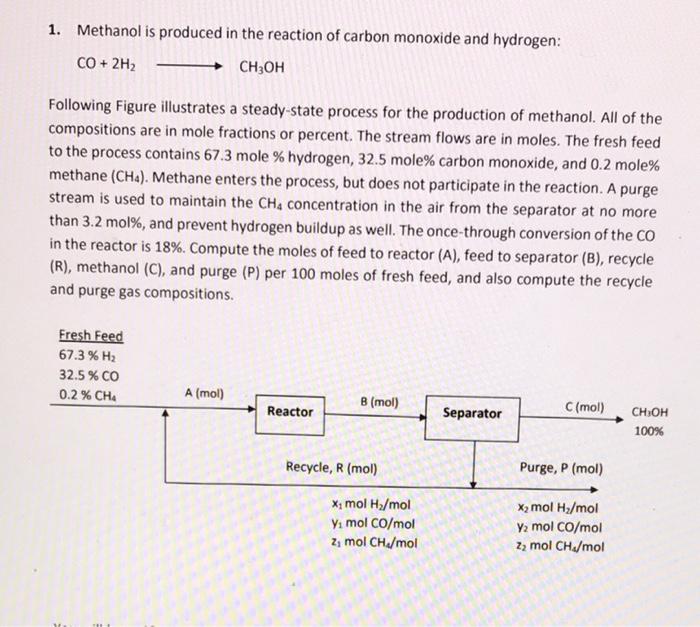
1. Methanol is produced in the reaction of carbon monoxide and hydrogen: CO + 2H2 CH3OH Following Figure illustrates a steady-state process for the production of methanol. All of the compositions are in mole fractions or percent. The stream flows are in moles. The fresh feed to the process contains 67.3 mole % hydrogen, 32.5 mole% carbon monoxide, and 0.2 mole% methane (CH). Methane enters the process, but does not participate in the reaction. A purge stream is used to maintain the CH4 concentration in the air from the separator at no more than 3.2 mol%, and prevent hydrogen buildup as well. The once-through conversion of the CO in the reactor is 18%. Compute the moles of feed to reactor (A), feed to separator (B), recycle (R), methanol (C), and purge (P) per 100 moles of fresh feed, and also compute the recycle and purge gas compositions. Fresh Feed 67.3% H2 32.5% CO 0.2% CHA A (mol) B (mol) Reactor Separator C(mol) CH3OH 100% Recycle, R (mol) Purge, P (mol) Xmol H2/mol Ya mol CO/mol 2, mol CH/mol X2 mol H2/mol Y2 mol CO/mol 22 mol CH./mol 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


