Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please explain shorly Iron reacts with chlorine to form iron(III) chloride according to the following reaction. 2Fe(s) + 3Cl2(g) 2FeCl3(s) Fe(s) Cl2(g) FeCl3(s) Initial Amount
Please explain shorly 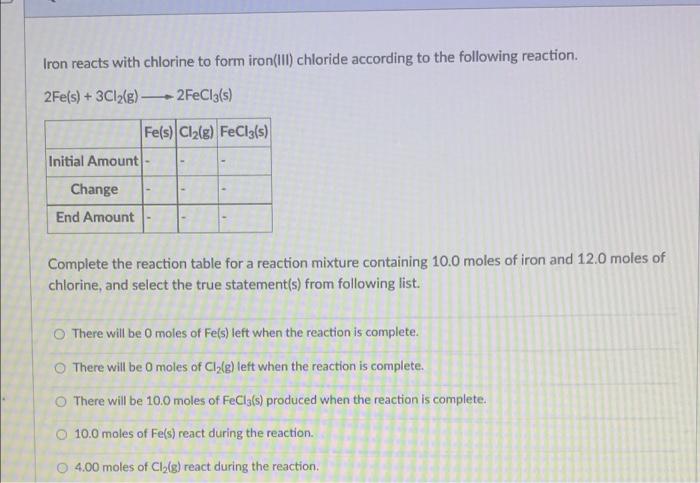
Iron reacts with chlorine to form iron(III) chloride according to the following reaction. 2Fe(s) + 3Cl2(g) 2FeCl3(s) Fe(s) Cl2(g) FeCl3(s) Initial Amount Change End Amount Complete the reaction table for a reaction mixture containing 10.0 moles of iron and 12.0 moles of chlorine, and select the true statement(s) from following list. There will be moles of Fe(s) left when the reaction is complete. There will be o moles of Cile) left when the reaction is complete. There will be 10.0 moles of FeCla(s) produced when the reaction is complete. O 10.0 moles of Fe(s) react during the reaction. 4.00 moles of Cl (g) react during the reaction 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


