Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please explain them. and An absorption band at 2820 and 2720cm1 due to the aldehyde CH stretching vibration would be present for the heptane and
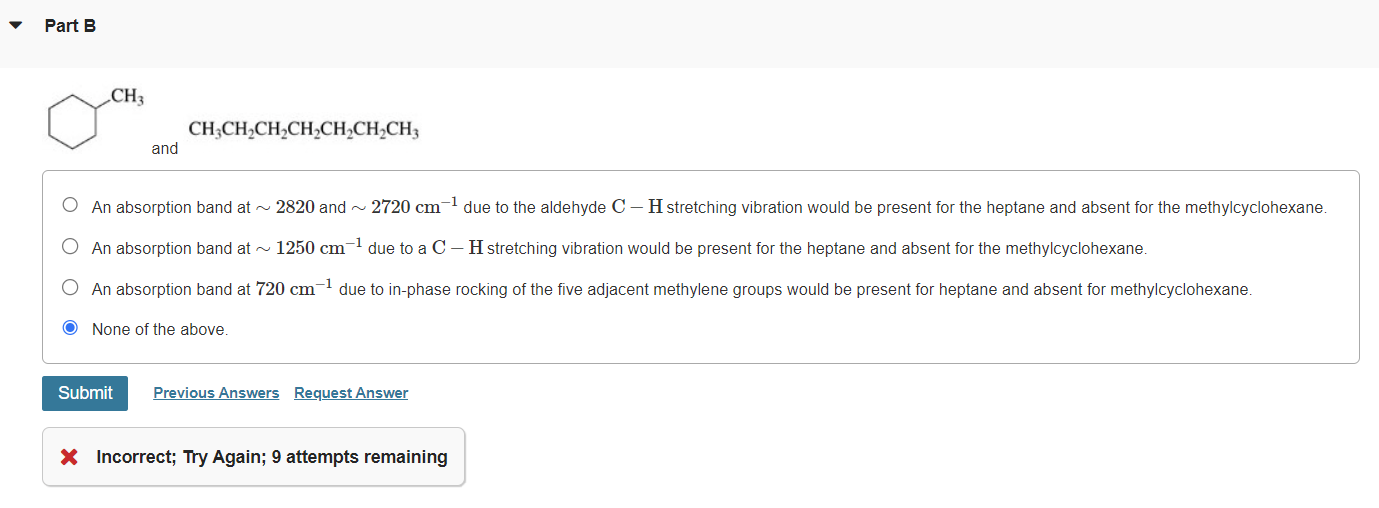


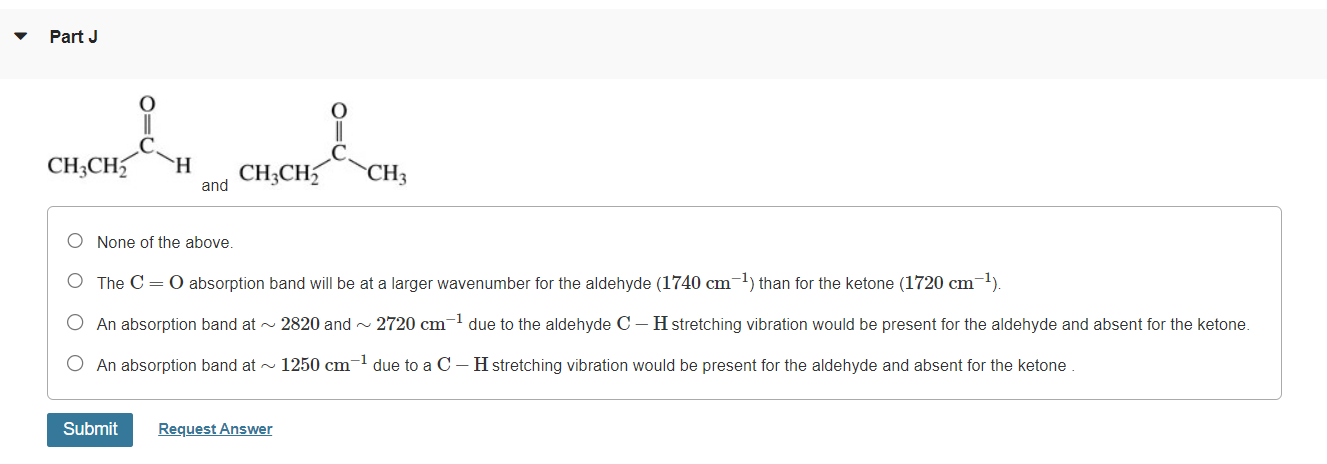
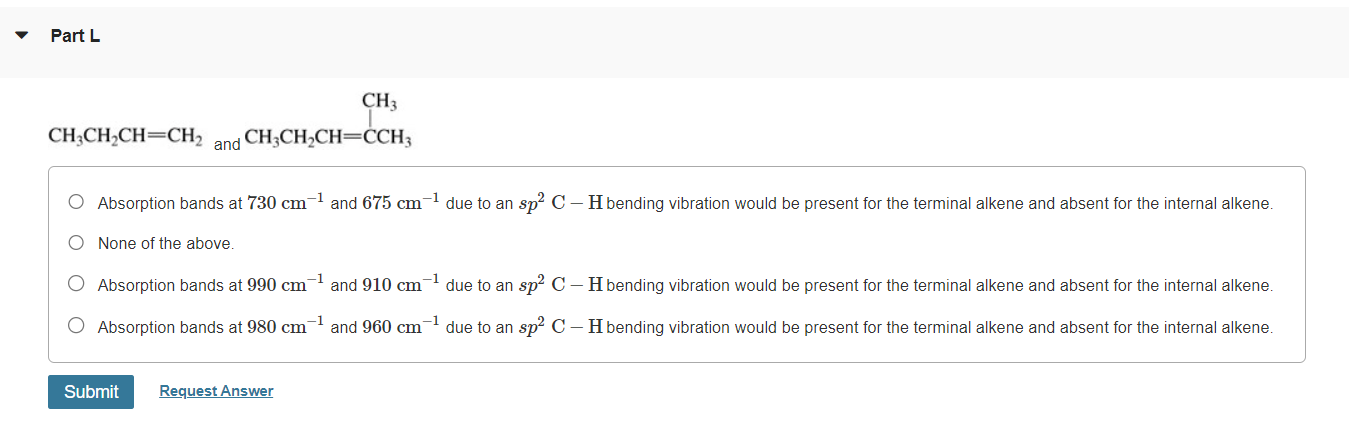 Please explain them.
Please explain them.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


