Question
Please fill in the following table. Thanks for all the help! Give the pKa and pKb of weak acids and bases. For each solution, calculate
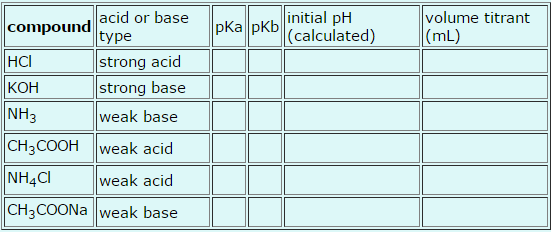
Please fill in the following table. Thanks for all the help!
Give the pKa and pKb of weak acids and bases. For each solution, calculate the pH of a 0.1000 M solution, and calculate the volume of 0.1000 M NaOH or HCl that would be required to titrate a 10.00 mL sample to the equivalence point. To fill-in the table below, answer the subsequent questions.
pKb(NH3) =
pKa(CH3COOH) =
pKa(NH4Cl) =
pKb(CH3COONa) =
pH(0.1000 M HCl) =
pH(0.1000 M KOH) =
pH(0.1000 M NH3) =
pH(0.1000 M CH3COOH) =
ptspH(0.1000 M NH4Cl) =
pH(0.1000 M CH3COONa) =
Volume of 0.1000 M NaOH required to titrate 10.00 mL of 0.1000 M HCl =
Volume of 0.1000 M HCl required to titrate 10.00 mL of 0.1000 M KOH =
Volume of 0.1000 M HCl required to titrate 10.00 mL of 0.1000 M NH3 =
Volume of 0.1000 M NaOH required to titrate 10.00 mL of 0.1000 M CH3COOH =
Volume of 0.1000 M NaOH required to titrate 10.00 mL of 0.1000 M NH4Cl =
Volume of 0.1000 M HCl required to titrate 10.00 mL of 0.1000 M CH3COONa =
acid or base type strong acid strong base weak base weak acid weak acid CH3COONa weak base compound HCI KOH NH3 CH3COOH NH4Cl pka pkb initial pH (calculated) volume titrant (mL)
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
1 pKb NH3475 2 pKaCH3COOH476at 25 degree Centigrade 3 pKa NH4Cl Does not exist as NH4Cl is a salt fo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


