Please fill out the last 2 remaining boxes in question 5. Please show the work for calculating the concentration and the uncertainty of the unknown sample. Thank you! If you can also do 6,7, or 8 that would be so helpful!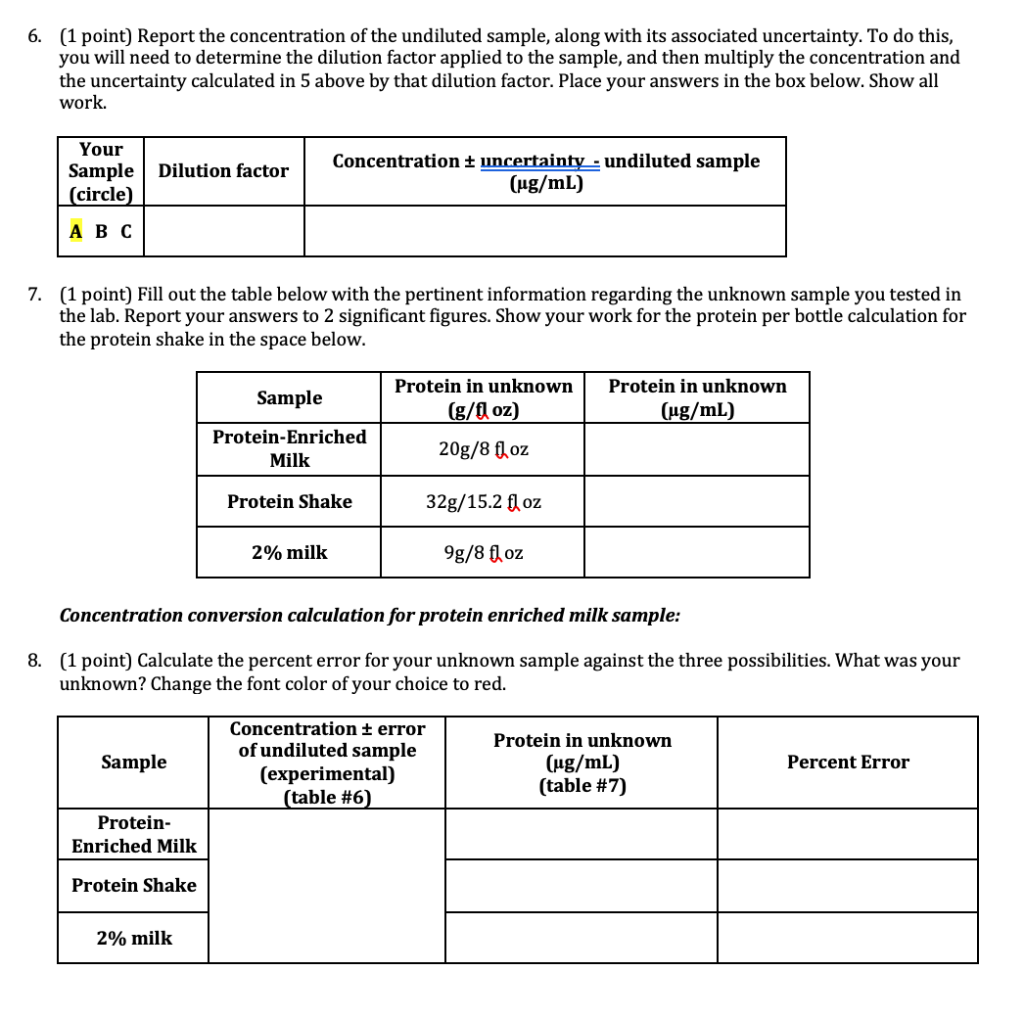
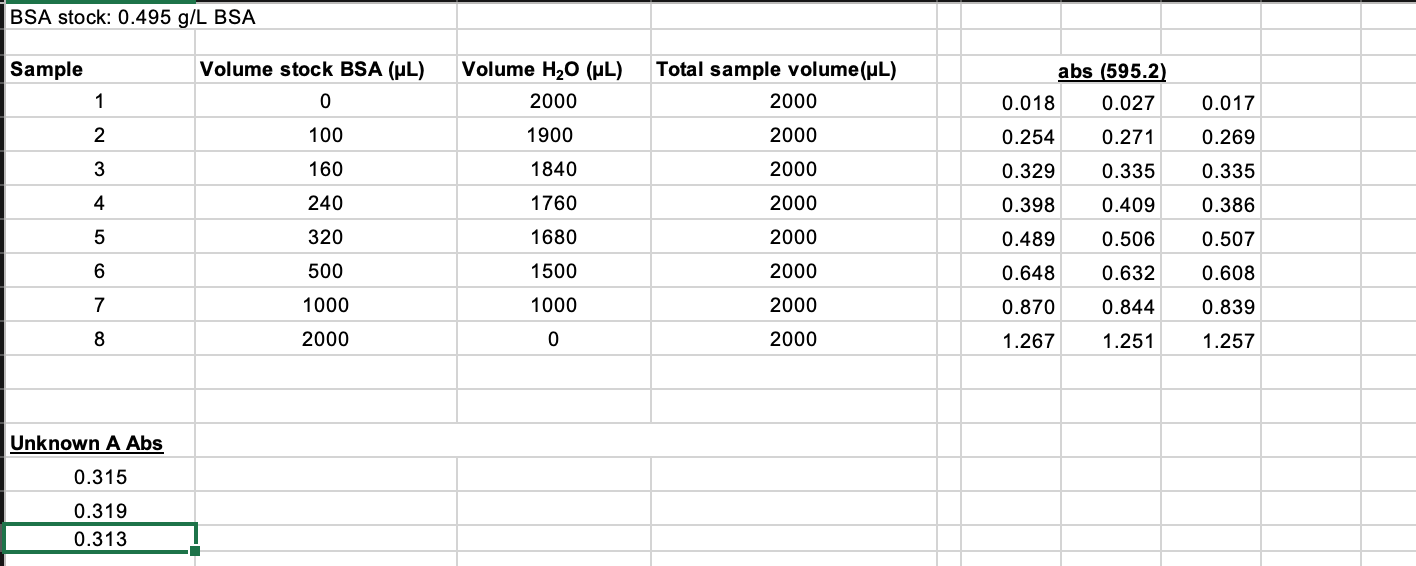
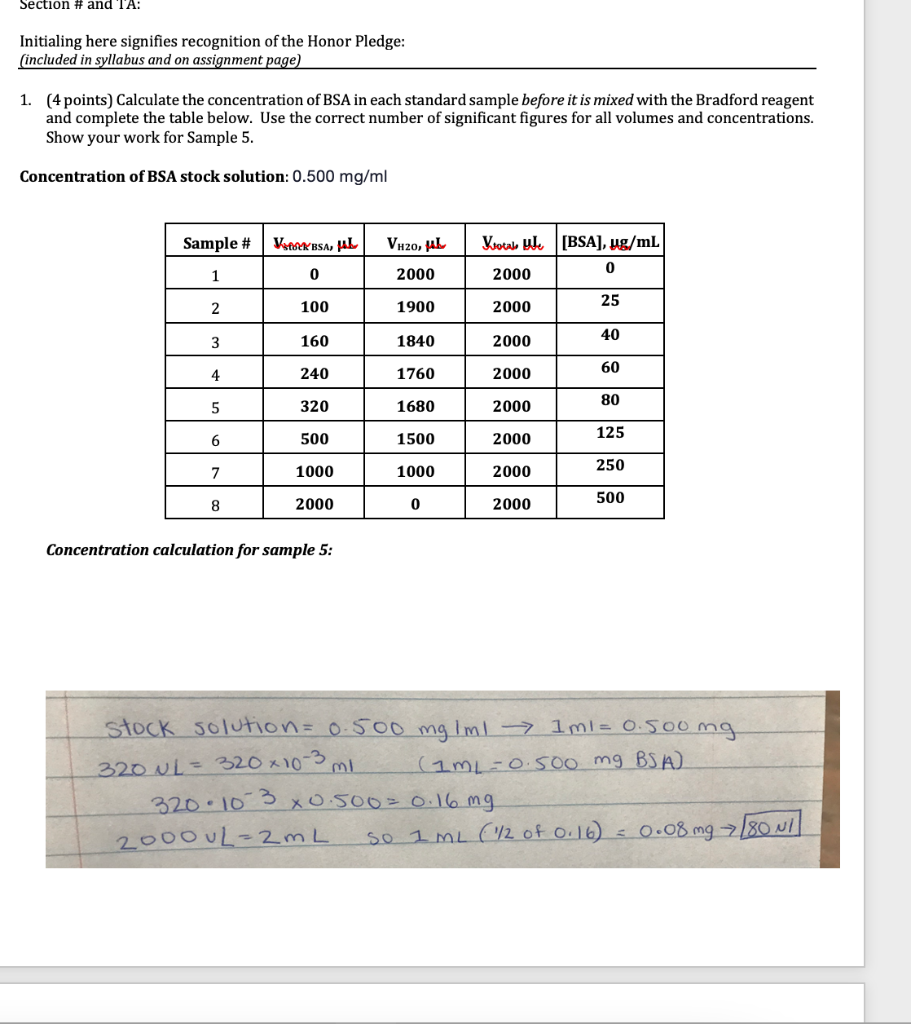

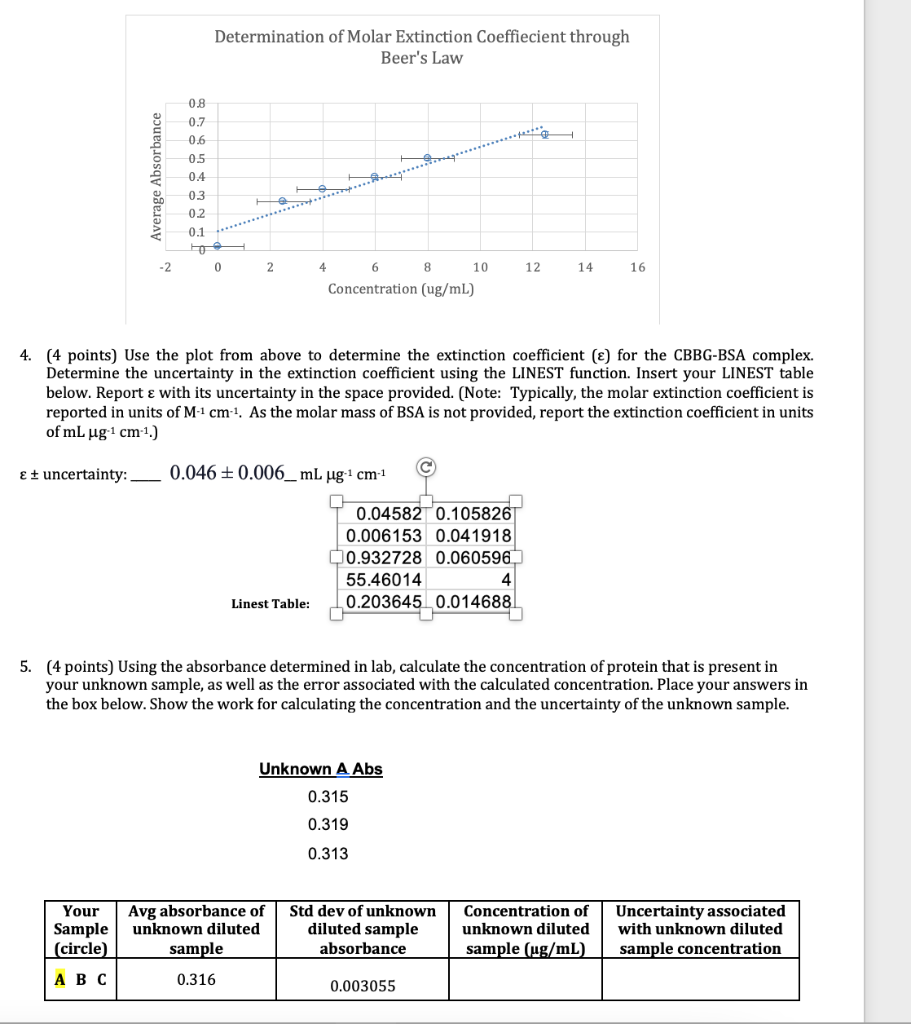
6. (1 point) Report the concentration of the undiluted sample, along with its associated uncertainty. To do this, you will need to determine the dilution factor applied to the sample, and then multiply the concentration and the uncertainty calculated in 5 above by that dilution factor. Place your answers in the box below. Show all work. Your Sample (circle) Dilution factor Concentration uncertainty - undiluted sample (ug/mL) 7. (1 point) Fill out the table below with the pertinent information regarding the unknown sample you tested in the lab. Report your answers to 2 significant figures. Show your work for the protein per bottle calculation for the protein shake in the space below. Sample Protein in unknown (g/fl oz) Protein in unknown (ug/mL) Protein-Enriched Milk 20g/8 fl oz Protein Shake 32g/15.2 fl oz 2% milk 9g/8 fl oz Concentration conversion calculation for protein enriched milk sample: 8. (1 point) Calculate the percent error for your unknown sample against the three possibilities. What was your unknown? Change the font color of your choice to red. Sample Concentration error of undiluted sample (experimental) (table #6) Protein in unknown (ug/mL) (table #7) Percent Error Protein- Enriched Milk Protein Shake 2% milk BSA stock: 0.495 g/L BSA Sample Volume stock BSA (UL) 0 1 Volume H2O (UL) 2000 1900 1840 0.017 Total sample volume(ul) 2000 2000 2000 abs (595.2) 0.018 0.027 0.254 0.271 2 100 0.269 3 160 0.329 0.335 0.335 4 240 1760 2000 0.398 0.409 0.386 5 320 1680 2000 0.506 0.507 0.489 0.648 6 500 1500 2000 0.632 0.608 7 1000 1000 2000 0.870 0.844 0.839 8 2000 0 2000 1.267 1.251 1.257 Unknown A Abs 0.315 0.319 0.313 Section # and TA: Initialing here signifies recognition of the Honor Pledge: (included in syllabus and on assignment page) 1. (4 points) Calculate the concentration of BSA in each standard sample before it is mixed with the Bradford reagent and complete the table below. Use the correct number of significant figures for all volumes and concentrations. Show your work for Sample 5. Concentration of BSA stock solution: 0.500 mg/ml Sample # WP_KBSA, HAL VH2O, LL Ktotale ul. [BSA), ug/mL 0 2000 1 0 2000 2 100 1900 2000 25 3 160 1840 2000 40 4 240 1760 2000 60 80 5 320 1680 2000 500 6 1500 2000 125 7 1000 1000 250 2000 8 2000 0 2000 500 Concentration calculation for sample 5: stock solution=0.500 mg Iml - 1 ml=0.500mg 320 L = 320 x10-3 (1mL-0.500 mg BSA) 320.10 3 X0.500=0.16 mg 2000uL=2mL so 1 ml (1/2 of 0.16) - 0.08 mg >[80 NI 2. (2 points) Calculate the concentration of BSA in the cuvette (after it has been mixed with CBBG) for each sample. Determine the average absorbance for each sample and the standard deviation in the absorbance for each sample and report in the table below. Sample # [BSA]cuvette ug/mL Average abs abs + standard deviation 1 0 0.021 0.025 2 2.5 0.265 0.272 3 4 0.333 0.336 6 4 6 0.398 0.407 5 8 0.501 0.509 12.5 0.629 0.645 7 25 0.851 0.864 8 50 1.258 1.264 3. (2 points) Using the data above, make a Beer's Law standard curve plot. a. Fit the data with a linear trendline. If any data points extend beyond the linear range, you can omit them from the graph. You must include rationale as to why the point(s) extended beyond the linear range to omit them. b. Use the standard deviations calculated in question 2 as error bars on your absorbance measurements. If your error bars cannot be seen, change the fill on your points to no fill so that they can be seen. c. Insert your graph into this document. Determination of Molar Extinction Coeffiecient through Beer's Law 1.6 14 1.2 1 Average Absorbance 0.8 0.6 0.4 02 - 10 0 10 40 50 60 20 30 Concentration (ug/ml) I would exclude the last two data points because they are outside my linear range and would skew the slope. In the next graph I excluded these points. I was unsure how to delete data points so I started all over. Determination of Molar Extinction Coeffiecient through Beer's Law Average Absorbance 0.8 0.7 0.6 05 0.4 03 0.2 0.1 -2 0 2 4 6 8 10 12 14 16 Concentration (ug/mL) 4. (4 points) Use the plot from above to determine the extinction coefficient (E) for the CBBG-BSA complex. Determine the uncertainty in the extinction coefficient using the LINEST function. Insert your LINEST table below. Report with its uncertainty in the space provided. (Note: Typically, the molar extinction coefficient is reported in units of M-1 cm 1. As the molar mass of BSA is not provided, report the extinction coefficient in units of mL ug 1 cm 1.) E + uncertainty: 0.046 + 0.006_mL ug-cm-1 0.04582 0.105826 0.006153 0.041918 0.932728 0.060596 55.46014 4 0.203645 0.014688 Linest Table: 5. (4 points) Using the absorbance determined in lab, calculate the concentration of protein that is present in your unknown sample, as well as the error associated with the calculated concentration. Place your answers in the box below. Show the work for calculating the concentration and the uncertainty of the unknown sample. Unknown A Abs 0.315 0.319 0.313 Your Sample (circle) Avg absorbance of unknown diluted sample Std dev of unknown diluted sample absorbance Concentration of unknown diluted sample (ug/mL) Uncertainty associated with unknown diluted sample concentration 0.316 0.003055 6. (1 point) Report the concentration of the undiluted sample, along with its associated uncertainty. To do this, you will need to determine the dilution factor applied to the sample, and then multiply the concentration and the uncertainty calculated in 5 above by that dilution factor. Place your answers in the box below. Show all work. Your Sample (circle) Dilution factor Concentration uncertainty - undiluted sample (ug/mL) 7. (1 point) Fill out the table below with the pertinent information regarding the unknown sample you tested in the lab. Report your answers to 2 significant figures. Show your work for the protein per bottle calculation for the protein shake in the space below. Sample Protein in unknown (g/fl oz) Protein in unknown (ug/mL) Protein-Enriched Milk 20g/8 fl oz Protein Shake 32g/15.2 fl oz 2% milk 9g/8 fl oz Concentration conversion calculation for protein enriched milk sample: 8. (1 point) Calculate the percent error for your unknown sample against the three possibilities. What was your unknown? Change the font color of your choice to red. Sample Concentration error of undiluted sample (experimental) (table #6) Protein in unknown (ug/mL) (table #7) Percent Error Protein- Enriched Milk Protein Shake 2% milk BSA stock: 0.495 g/L BSA Sample Volume stock BSA (UL) 0 1 Volume H2O (UL) 2000 1900 1840 0.017 Total sample volume(ul) 2000 2000 2000 abs (595.2) 0.018 0.027 0.254 0.271 2 100 0.269 3 160 0.329 0.335 0.335 4 240 1760 2000 0.398 0.409 0.386 5 320 1680 2000 0.506 0.507 0.489 0.648 6 500 1500 2000 0.632 0.608 7 1000 1000 2000 0.870 0.844 0.839 8 2000 0 2000 1.267 1.251 1.257 Unknown A Abs 0.315 0.319 0.313 Section # and TA: Initialing here signifies recognition of the Honor Pledge: (included in syllabus and on assignment page) 1. (4 points) Calculate the concentration of BSA in each standard sample before it is mixed with the Bradford reagent and complete the table below. Use the correct number of significant figures for all volumes and concentrations. Show your work for Sample 5. Concentration of BSA stock solution: 0.500 mg/ml Sample # WP_KBSA, HAL VH2O, LL Ktotale ul. [BSA), ug/mL 0 2000 1 0 2000 2 100 1900 2000 25 3 160 1840 2000 40 4 240 1760 2000 60 80 5 320 1680 2000 500 6 1500 2000 125 7 1000 1000 250 2000 8 2000 0 2000 500 Concentration calculation for sample 5: stock solution=0.500 mg Iml - 1 ml=0.500mg 320 L = 320 x10-3 (1mL-0.500 mg BSA) 320.10 3 X0.500=0.16 mg 2000uL=2mL so 1 ml (1/2 of 0.16) - 0.08 mg >[80 NI 2. (2 points) Calculate the concentration of BSA in the cuvette (after it has been mixed with CBBG) for each sample. Determine the average absorbance for each sample and the standard deviation in the absorbance for each sample and report in the table below. Sample # [BSA]cuvette ug/mL Average abs abs + standard deviation 1 0 0.021 0.025 2 2.5 0.265 0.272 3 4 0.333 0.336 6 4 6 0.398 0.407 5 8 0.501 0.509 12.5 0.629 0.645 7 25 0.851 0.864 8 50 1.258 1.264 3. (2 points) Using the data above, make a Beer's Law standard curve plot. a. Fit the data with a linear trendline. If any data points extend beyond the linear range, you can omit them from the graph. You must include rationale as to why the point(s) extended beyond the linear range to omit them. b. Use the standard deviations calculated in question 2 as error bars on your absorbance measurements. If your error bars cannot be seen, change the fill on your points to no fill so that they can be seen. c. Insert your graph into this document. Determination of Molar Extinction Coeffiecient through Beer's Law 1.6 14 1.2 1 Average Absorbance 0.8 0.6 0.4 02 - 10 0 10 40 50 60 20 30 Concentration (ug/ml) I would exclude the last two data points because they are outside my linear range and would skew the slope. In the next graph I excluded these points. I was unsure how to delete data points so I started all over. Determination of Molar Extinction Coeffiecient through Beer's Law Average Absorbance 0.8 0.7 0.6 05 0.4 03 0.2 0.1 -2 0 2 4 6 8 10 12 14 16 Concentration (ug/mL) 4. (4 points) Use the plot from above to determine the extinction coefficient (E) for the CBBG-BSA complex. Determine the uncertainty in the extinction coefficient using the LINEST function. Insert your LINEST table below. Report with its uncertainty in the space provided. (Note: Typically, the molar extinction coefficient is reported in units of M-1 cm 1. As the molar mass of BSA is not provided, report the extinction coefficient in units of mL ug 1 cm 1.) E + uncertainty: 0.046 + 0.006_mL ug-cm-1 0.04582 0.105826 0.006153 0.041918 0.932728 0.060596 55.46014 4 0.203645 0.014688 Linest Table: 5. (4 points) Using the absorbance determined in lab, calculate the concentration of protein that is present in your unknown sample, as well as the error associated with the calculated concentration. Place your answers in the box below. Show the work for calculating the concentration and the uncertainty of the unknown sample. Unknown A Abs 0.315 0.319 0.313 Your Sample (circle) Avg absorbance of unknown diluted sample Std dev of unknown diluted sample absorbance Concentration of unknown diluted sample (ug/mL) Uncertainty associated with unknown diluted sample concentration 0.316 0.003055











