Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please fill up all with showing calculations Titration Data Calculations Moles of Na2S2O3 in titration Moles of NaOCl in titration Moles of undiluted NaOCl Mass
please fill up all with showing calculations 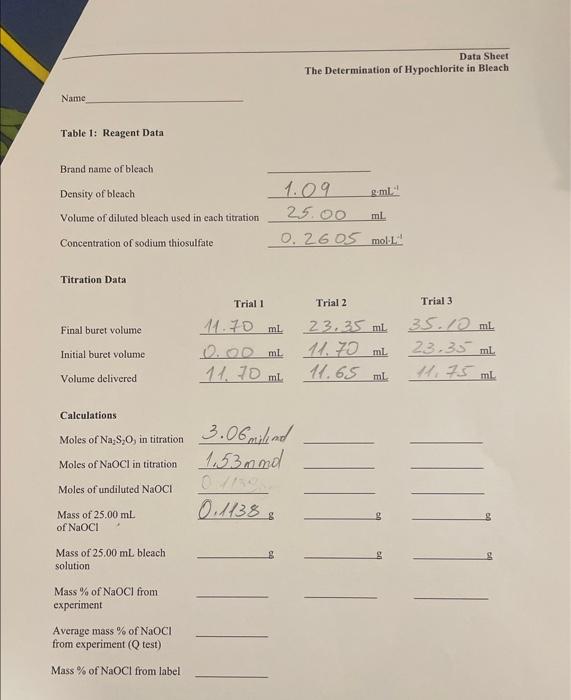




Titration Data Calculations Moles of Na2S2O3 in titration Moles of NaOCl in titration Moles of undiluted NaOCl Mass of 25.00mL of NaOCl Mass of 25.00mL bleach solution Mass % of NaOCl from experiment Average mass % of NaOCl from experiment ( Q test) Mass % of NaOCl from label The Determination of Hypochlorite in Bleach. Reading assignment: Chang, Chemistry 10 edition, pages 156-159. We will stady an example of a redox titration in order to determine the concentration of sodium hypochlorite, the active ingredient in commercial bleach. Safety Note: Safety glasses are required when performing this experiment 50.00-mL buret, 250 -mL volumetric flask, 25.00-mL volumetric pipette, pipette pump, 300 -mL Erlenmeyer flask, 10-mL graduated cylinder, 100 -mL graduated cylinder, 100 -mL beaker, commercial bleach solution (4-6\% hypochlorite), 10% potassium iodide solution ( KI),2M bydrochloric acid solution (HCl), 0,26M sodium thiosulfate solution (Na3S2O4), starch solution. An aqueous solution of sodium hypochlorite (NaOCl) is a clear, slightly yellow liquid, and is commonly known as bleach. Aside from its uses as a bleaching agent, sodium hypochlorite solutions are also used as sterilizing agents and in water treatment. Industrial uses include agriculture, food, paper production, and textiles. Sodium hypochlorite is also added to waste water to reduce odors. The concentration of sodium hypochlorite in bleach solutions can be determined by titration. A desirable method would be to find a titrant that reacts with NaOCl to form a colored product. But there are no known titrant-indicator systems that work well. Therefore, we must use a multi-step method to titrate sodium hypochlorite. In the first step sodium hypochlorite, hydrochloric acid, iodide ion, and starch are combined to form a starch-triodide complex. In this step there are three reactions that take place: (1) Hydrochloric acid reacts with sodium hypochlorite to form hypochlorous acid: NaOCl(aq)+HCl(aq)HOCl(aq)+NaCl(aq) (2) Hypochlorous acid reacts with iodide when the solution is acidic: HOCl(aq)+HCl(aq)+3I(aq)I3(aq)+2Cl+(aq)+H2O(l) Tridiodide, I3, is a dark red complex. A dark blue complex is formed when triiodide is combined with starch. (3) Ij+ starch [Ij][ starch ] The result of these three reactions is that when sodium hypochlorite is present the starch-triodide complex is produced. This is useful because the result of these three reactions is the formation of a dark. blue complex that has a concentration that is proportional to the amount of sodium hypochlorite in the solution. In the next step, the starch-triodide product is titrated by sodium thiosulfate to form a colorless solution of iodide, dithionate, and uncomplexed starch: If iodide is added in excess to the hypochlorous acid then all of the hypochlorous acid will be reacted. forming the dark blue starch-trijodide complex. The bypochlorite acts as a limiting reagent, determining brw mach triodide is produced. We can then titrate the triodide-starch complex with the thicsulfite to deernine the concentration of the complex formed. This can then be uscd to calculate the initial. concestration of hypochlorite. SAFETY PRECAUTIONS Safcty ghasses or goggles must be wom throughout this experiment. Bleach is a strong oxidoring agcot and should be washed from skin. Bleach can also damage clothing. For that reason, take care not to splash any of the bleach solution on clothine. Students perfom titrations individually. Dilutioa of the bleach (Step 2) can be perfortecd in pain. 1. Obtain 60mL of sodium thiosulfate solution wsing a clean 100 -mL beaker. Recond the ooncentration of this solution on the Data Shect. Rinse a 50 -ml. buret with tap water and then digtilled or deionired water. Rinse the buret with a few milliliters of the sodium thiosuifate solution. Fill the buret to just above the 0mL mark with sodrum thiosulfate solution. Allow a few millliters to pour throegh the buret. tip so that any trapped air can be flushed through. Read and recoed the initial buret level to the nearest: 0.05mL 2. Performa a 10-fold quaatitative dilution of bleach. This step can be perfotrined with a partaer and the diluted bleach shared. Use a clean 25-ml. volumatric pipet to deliver 25.00ml. of bleach nolution into a clean 250 -ml. volumctrie flask. Add distilled or deionined water to the mark of the volumetric flask. Be sure to mix the solution well to ensure that it is hocnogencous. The bleach shoold now be eac-tenth its original concentration and can be used in your titrations. Rinse the pipet with distilled or delocized water to remove the bleach solution. 3. The titration takes place in a 300 -imL Eilenencyer flavk which will be called the reaction flask. Using the 25 -mhl wolumetrie pipet, carefully add 25.00mL of dilated bleach (from step 2) to the reaction flask. 4. Using a 100-ml. graduated cylinder, add about 15ml of distilled or deionired water to the reaction flask. 5. Using a 100-mL graduated cylinder, add about 20mL of 10% potassium iodide solution to the teaction flask. This 10%KI solution should be clear, and not ycllow. If the solution is yellow then it is likely aritaminated. 6. Pour about 2mL of 5 tarch solution in a 10-mL graduated cylinder and place the cylinder next to the reaction flask. This starch solution will be added to the flask later. 7. Using a 100-mL graduated cylinder, add about 20mL of hydrochloric acid solution to the reaction flask. Begin the titration promptly after adding this solution to the mixture. 8. Begin titrating the bleach solution with sodium thiosulfate solution. Allow about 23mL of the thiosulfate solution to run into the reaction flask and close the stopcock. Don't write the volume down at this point. You'll be adding more thiosulfate in a few moments. There may be a dark precipitate of crystals of solid iodine if you are slow in getting to this stage. It should dissolve and react with the thiosulfate solution by mixing the solution. 8. Add the 2mL of starch solution from the graduated cylinder to the reaction flask. The color of the solution should now be dark blue. 9. Continue the titration dropwise with constant swirling until the solution becomes clear. This clear solution signals the endpoint of the titration. Record the buret volume level in the data sheet to the nearest 0.05mL. The contents of the reaction flask may be washed into the drain. 10. Perform two more titrations by repeating steps 39. 11. After completing your final titration pour any unused reagents down the drain. The buret should be rinsed with distilled or deionized water. All glassware should be returned to the appropriate location. To calculate the molarity of the sodium hypochlorite solution we use the titration volume, molarity of the thiosulfate titrant, stoichiometry of the reactions, and volume of the sample of diluted bleach solution. 1. Calculate the number of moles of titrant (sodium thiosulfate) for each titration: moles Na2S2O3=( molarity of Na2S2O3 solution ) (volume of Na2S2O3 solution) 2. The moles of hypochlorite are found from the stoichiometry of the reaction with thiosulfate. The stoichiometry of the equation shows that there are two moles of thiosulfate ion per mole of hypochlorous acid. Note that the moles of HOCl are equal to the moles of NaOCl and OCl. H+(aq)+HOCl(aq)+2S2O32(aq)S4O62(aq)+Cl(aq)+H2O(aq) moles HOCl= moles S2O32(2molS2O321molHOCl) 3. The bleach solution that we titrated was diluted by a factor of 10 . Multiply the moles of the hypochlorous acid by 10 to take this factor into account. This will give the number of moles of HOCl in 25.00mL of undiluted bleach. 4. Sodium hypochlorite (NaOCl) is the form of the hypochlorite that is reported on bleach bottles, not hypochlorous acid. The sodium hypochlorite mass percent is found from the mass of sodium hypochlorite in 25.00mL and the mass of the 25.00 bleach solution: mass %=(massofbleachsolutionmassofNaOCl)100 5. The mass of the NaOCl is found from the moles of NaOCl and the molar mass of NaOCl : mass of NaOCl=( moles of NaOCl)( molar mass of NaOCl) 6. The mass of 25.00mL of bleach solution is found from its density and the volume (25.00mL) : mass of bleach solution =( density of bleach ) (volume of solution) 7. Perform a Q test on your mass percent values and calculate an average mass percent concentration. How close is your result to the value listed on the bottle? Qoale=rangegap m95\% confidence Observations and Notes The Determination of Hypochlorite in Bleach Date 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


