Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please fill your answer inside the table and explain your answer by writing one paragraph in the discussion explanation part.Thx Section II: Functional Group Tests
Please fill your answer inside the table and explain your answer by writing one paragraph in the discussion explanation part.Thx 


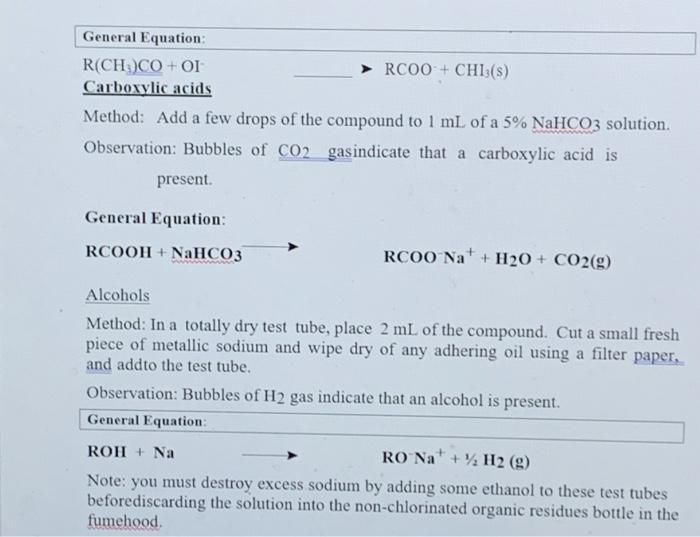
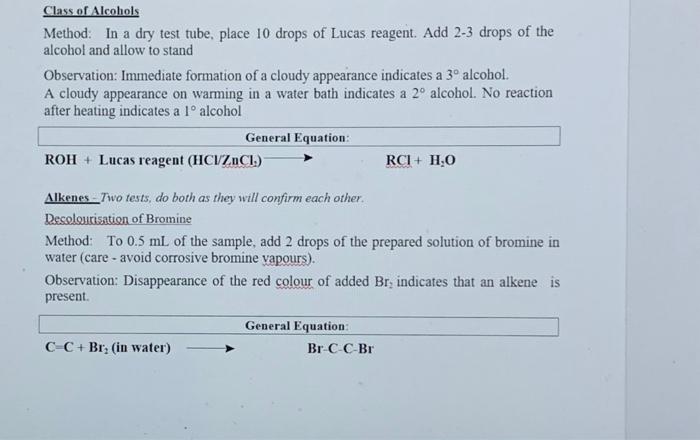

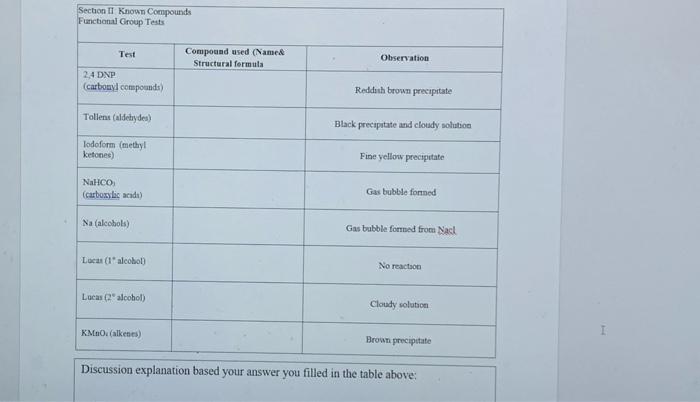
Section II: Functional Group Tests Additional Safety Notes: Do all tests in the fumehood Don't get 2,4-dinitrophenylhydrazine (2.4-DNP) reagent on your skin. The solid (although not the solution) has been shown to be very strongly mutagenic i.e. it causes genetic damage. Sodium metal is very reactive; keep it well away from water and wet glassware. Destroy any excess sodium in any test tubes used in the alcohol, tests in Parts A and B by adding some ethanol. It reacts with sodium metal, but in a less rigorous or more controlled manner than water. Bromine yapour is corrosive. Handle the solution in the fumsbood A: Known Compounds It is most important that you carefully follow the procedure specified for each test, otherwise results will inconclusive or incorrect. To familiarise yourself with the appearance of a positive result, you are to carry out the tests on known examples of eachof the compounds before starting on the unknowns. The known and unknowns are all liquids because the tests are more easily performed on liquids, however the tests can also be done on solids, provided an inert solvent is used. Representative known examples of the following compounds are supplied in thefuchged testing stations: Aldehydes Methyl ketones Non-methyl ketones Carboxylic acids 1, 2 and 3 alcohols Alkenes Alkanes Choose the example of each class and carry out the appropriate functional group test to familiariss yourself with what a positive result looks like. See the descriptions given below for each test. Note down the name and structural formula of the compound you use for each test on the report sheet. Write down any observations and conclusions for the known compounds on the reportsheet. Write chemical equations in the general form in the spaces provided. RETAIN THE TEST-TUBES SHOWING THE POSITIVE RESULT FOR EACH CLASS OF COMPOUND - if you do not, you will have to repeat the test series again and it reflects that you did not read the method section thoroughly Show your test tubes to your demonstrator. You will be asked to describe what you did, what you observed and the conclusion that you reached You are required in other words to verbally communicate your experiment for this section. Marks will be allocated for your attempt to express yourself using chemical names of compounds and terminology associated with the method Once you have adequately explained your experimental method and outcomes to the demonstrator you will be given your 1 unknown compound. Functional Group Tests Carbonyl Compounds Method: Place 1 mL of 2,4-dinitrophenylhydrazine (2,4-DNP) reagentsolution into a clean, dry test tube (care - avoid skin contact). Add 2 or 3 drops of compound and allow to stand for several minutes Observation: A yellow, red or orange precipitate indicates the presence of a carbonyl group (Absence of a precipitate is a negative result, even if solutions coloured General Equation: R2CO + 2,4-DNP R2CN-NHG (where G - NO2 Ar-NO2) Aldehyder Method: In a very clean test tube, place I ml. of freshly prepared Tellin's reagent Add 2 or 3 drops of the compound, mix thoroughly and warm gently in a water bath Observation: A silver mirror indicates the presence of an aldehyde group (Sometimesblack colloidal silver precipitates out instead - also a positive result) General Equation: RCHO+ Tollen's reagent (Ag (2) *g() + RCOO Methyl Ketones Method: Place 2 mL of ~ 1M NaOH in a test tube, add drops of compound. Then add the iodine-potassium iodide reagent dropwise with shaking until a yellow colour (of 12) persists in solution. Observation: A fine yellow precipitate of iodoform (CHI3) indicates that a methyl ketoneis present. (A lack of precipitate is a negative result. Confirmation:If a precipitate is not formed, warm in a water bath (-60 C for 2 minutes) add more iodine-potassium iodide reagent. If necessary, excess yellow colour in the solutions can be removed with 1-2 drops of NaOH and diluting with 10 mL of coldwater. So that the precipitate, if present can be more clearly seen. I General Equation: R(CH)CO+OI RC00+CHIB(S) Carboxylic acids Method: Add a few drops of the compound to 1 mL of a 5% NaHCO3 solution. Observation: Bubbles of CO2 gasindicate that a carboxylic acid is present General Equation: RCOOH + NaHCO3 RCOO Na+ H2O + CO2(g) Alcohols Method: In a totally dry test tube, place 2 mL of the compound. Cut a small fresh piece of metallic sodium and wipe dry of any adhering oil using a filter paper. and addto the test tube. Observation: Bubbles of H2 gas indicate that an alcohol is present. General Equation ROH + Na RO Na+ H2(g) Note: you must destroy excess sodium by adding some ethanol to these test tubes beforediscarding the solution into the non-chlorinated organic residues bottle in the fumehood Class of Alcohols Method: In a dry test tube place 10 drops of Lucas reagent Add 2-3 drops of the alcohol and allow to stand Observation: Immediate formation of a cloudy appearance indicates a 3 alcohol. A cloudy appearance on warming in a water bath indicates a 2 alcohol. No reaction after heating indicates a l alcohol General Equation: ROH + Lucas reagent (HCIZACI) RCI+ H.O Alkenes - Two tests, do both as they will confirm each other. Decolourisation of Bromine Method: To 0.5 mL of the sample, add 2 drops of the prepared solution of bromine in water (care - avoid corrosive bromine vapours). Observation: Disappearance of the red colour of added Br, indicates that an alkene is present. General Equation C C + Brz (in water) Br CC-Br Decolourisation of Permanganate Method: To 0.5 mL of the sample, add 2 drops of dilute aqueous KMnO. Observation: Disappearance of the purple colour of added KMnO, and the appearance of a dark brown precipitate of Mno, indicates an alkene is present. General Equation: C-C + KMnO. (cold, dilute, neutral) HO-CCOH+MnO.(5) I Section II Known Compounds Functional Group Tests Test Compound used (Named Structural formula Observation 2.4 DNP (carbonyl compounds Reddish brown precipitate Tollent (aldehydes) Black precipitato and cloudy solution lodoform (methyl ketones) Fine yellow precipitate NaHCO (carboxylic acide) Gas bubble forned Na (alcohols) Gas bubble formed from Nack Local (alcohol No reaction Lucas Calcohol) Cloudy solution KMnO (alkenes) Brown precipitate Discussion explanation based your answer you filled in the table above 






Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


