Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please finish this with all the steps n-butanol is produced by the reaction of propylene, carbon monoxide and hydrogen according to the following reaction: CH3CH=CH2+CO+2H2CH3CH2CH2CH2OH
please finish this with all the steps 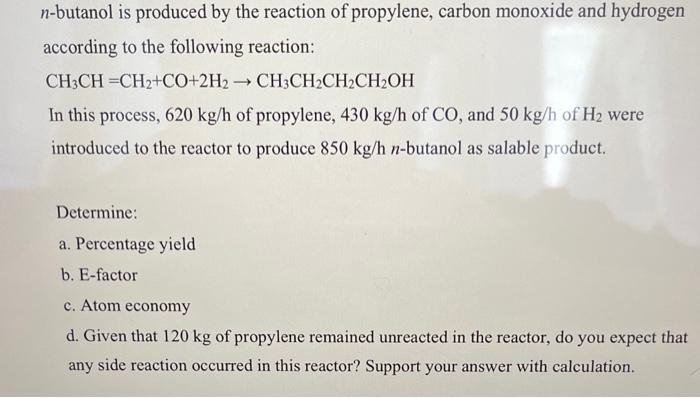
n-butanol is produced by the reaction of propylene, carbon monoxide and hydrogen according to the following reaction: CH3CH=CH2+CO+2H2CH3CH2CH2CH2OH In this process, 620kg/h of propylene, 430kg/h of CO, and 50kg/h of H2 were introduced to the reactor to produce 850kg/hn-butanol as salable product. Determine: a. Percentage yield b. E-factor c. Atom economy d. Given that 120kg of propylene remained unreacted in the reactor, do you expect that any side reaction occurred in this reactor? Support your answer with calculation 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


