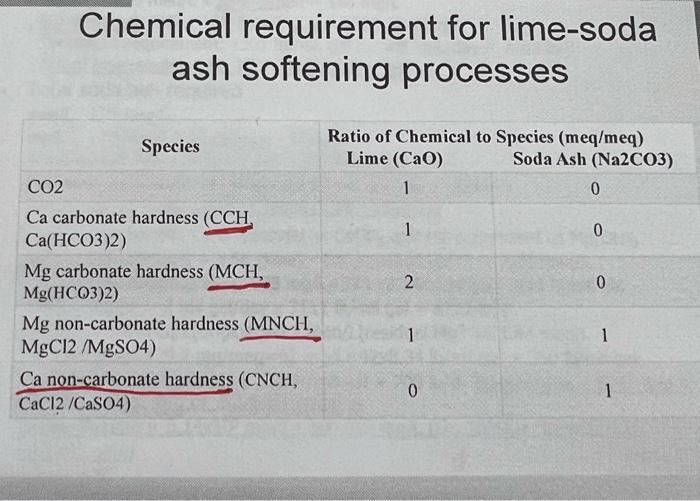
please follow the same steps as provided in the example.
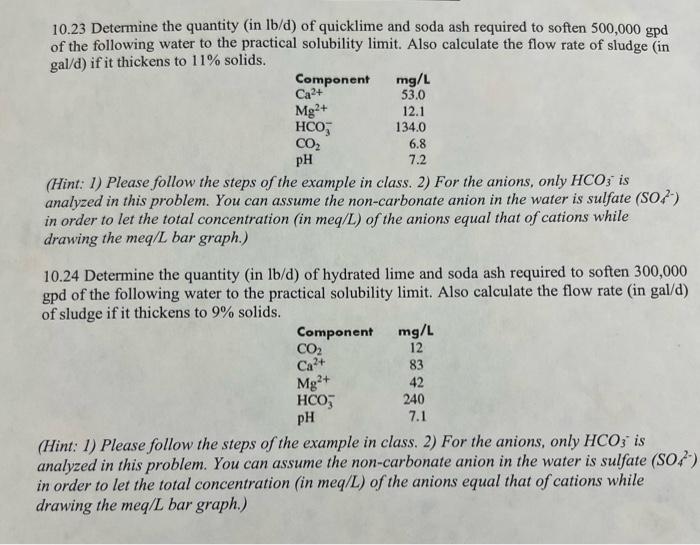
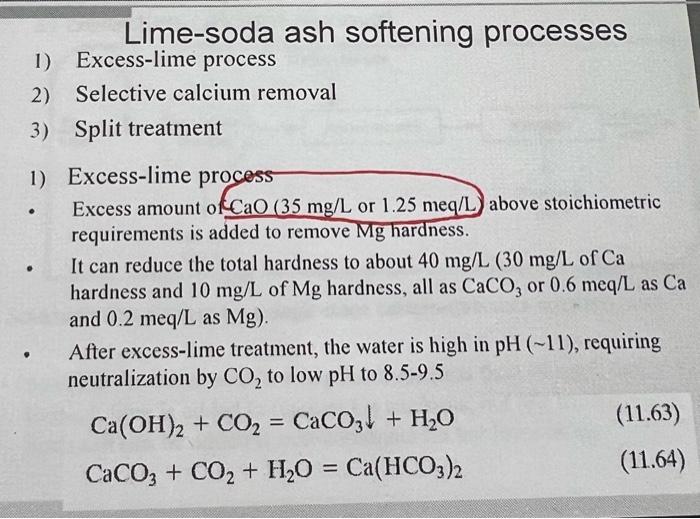
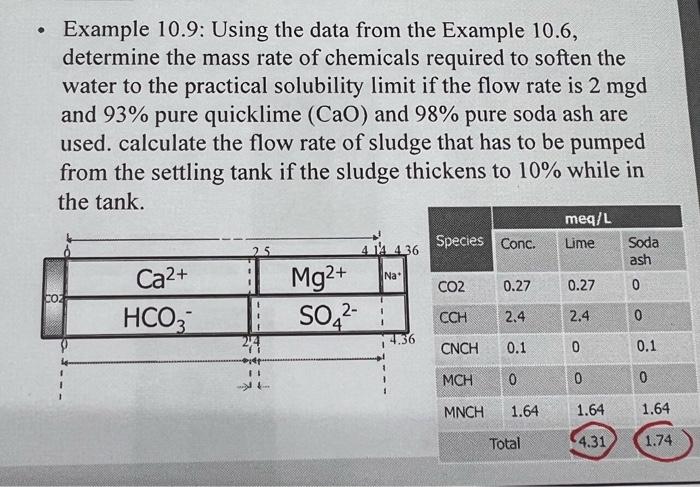
10.23 Determine the quantity (in lb/d) of quicklime and soda ash required to soften 500,000gpd of the following water to the practical solubility limit. Also calculate the flow rate of sludge (in gal/d) if it thickens to 11% solids. (Hint: 1) Please follow the steps of the example in class. 2) For the anions, only HCO3 is analyzed in this problem. You can assume the non-carbonate anion in the water is sulfate (SO42) in order to let the total concentration (in meq/L) of the anions equal that of cations while drawing the meq/L bar graph.) 10.24 Determine the quantity (in lb/d) of hydrated lime and soda ash required to soften 300,000 gpd of the following water to the practical solubility limit. Also calculate the flow rate (in gal/d) of sludge if it thickens to 9% solids. (Hint: 1) Please follow the steps of the example in class. 2) For the anions, only HCO3 is analyzed in this problem. You can assume the non-carbonate anion in the water is sulfate SO42, in order to let the total concentration (in meq/L) of the anions equal that of cations while drawing the meq/L bar graph.) Chemical requirement for lime-soda ash softening processes Lime-soda ash softening processes 1) Excess-lime process 2) Selective calcium removal 3) Split treatment 1) Excess-lime process - Excess amount of CaO(35mg/L or 1.25meq/L) above stoichiometric requirements is added to remove Mg hardness. - It can reduce the total hardness to about 40mg/L(30mg/L of Ca hardness and 10mg/L of Mg hardness, all as CaCO3 or 0.6meq/L as Ca and 0.2meq/L as Mg ). - After excess-lime treatment, the water is high in pH(11), requiring neutralization by CO2 to low pH to 8.59.5 Ca(OH)2+CO2=CaCO3+H2OCaCO3+CO2+H2O=Ca(HCO3)2 Example 10.9: Using the data from the Example 10.6, determine the mass rate of chemicals required to soften the water to the practical solubility limit if the flow rate is 2mgd and 93% pure quicklime (CaO) and 98% pure soda ash are used. calculate the flow rate of sludge that has to be pumped from the settling tank if the sludge thickens to 10% while in the tank. Total lime required meq /L:4.31meq/L+1.25meq/L (excess lime) =5.56meq/L mg/L:5.56meq/L28mg/meq=156mg/L lb/milliongal:1568.34lb/mil. gal. =1301lb/mil. gal. Treatment requirement: 1301lb/mil. gal 2mil. gal. /day=2602lb/d Total soda ash required 2602lb/d/0.93=2800lb/d mg/L:1.74meq/L53mg/meq=92mg/L lb/ million gal: 928.34lb/mil gal. =767lb/mil gal. Treatment requirement: 762lb/mil. gal x 2mil. gal. / day =1534lb/d Actual soda ash requirement: 1534lb/d/0.98=1565lb/d Sludge produced: Ca is removed as CaCO3 and Mg is removed as Mg(OH)2 CaCO3:2.5 (original) +5.56 (lime) 0.6( residual Ca2+)=7.46meq/L =7.46meq/L50mg/meq=373mg/L=3738.34lb/milgal=3111lb/mil gal CaCO3 sludge =2milgal/ day 3111lb/milgal=6222lb/d Mg(OH)2:1.64meq/L (original) 0.2meq/L( residual Mg2+)=1.44meq/L =1.44meq/L29.2mg/meq=42mg/L=428.34lb/milgal=350lb/milgal Mg sludge =2milgal/day350lb/milgal=700lb/d Total Sludge =6222lb/c 700lb/d=6922lb/d or 3.14109mg/d or 8300gal/d