please give an explanation for each :)
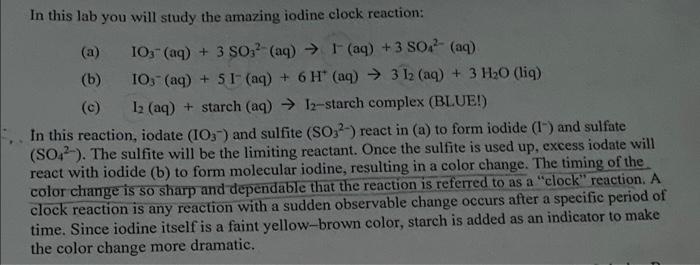
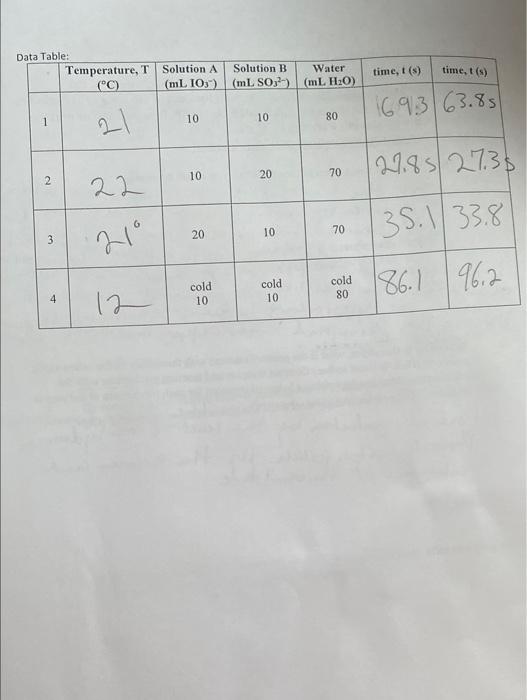
In this lab you will study the amazing iodine clock reaction: (a) IO3(aq) +3SO32 (aq) I(aq) +3SO42 (aq) (b) IO3(aq)+5I(aq)+6H+(aq) 3I2 (aq) +3H2O (liq) (c) I2 (aq) +starch(aq)I2-starch complex (BLUE!) In this reaction, iodate (IO3)and sulfite (SO32) react in (a) to form iodide (I)and sulfate (SO42). The sulfite will be the limiting reactant. Once the sulfite is used up, excess iodate will react with iodide (b) to form molecular iodine, resulting in a color change. The timing of the color change is so sharp and dependable that the reaction is referred to as a "clock" reaction. A clock reaction is any reaction with a sudden observable change occurs after a specific period of time. Since iodine itself is a faint yellow-brown color, starch is added as an indicator to make the color change more dramatic. 4. Write an experimental rate lavis for the teacong you stadied icday, after mouncievg off your reaction onders in the neures integer (orhalfinkeger). Post-lab Questions: 1. Identify all reactants, products and intermediates in the reaction you studied today, Recall that reactants are consumed in the overall reaction, products ate produced in the overall reaction, intermediates are produced in an carly step and then consumed in a later step, and catalysts are consumed in an early step and then produced in a later step. RCTS - 403,3SO32111 PDTS - 3SO4, 31+2012+L2 INTS - 2. By comparing the relative rates of reactions $1&2, determine the reaction order in [SO32]. Show your calculations, and show three significant figures in your final answer. 3. By comparing the relative rates of reactions \#1 \& 3, determine the reaction order in [IO3]. Show your calculations, and show three significant figures in your final answer. Write an experimental rate law for the reaction you studied today, after rounding off your reaction orders to the nearest integer (or half-integer). In this lab you will study the amazing iodine clock reaction: (a) IO3(aq) +3SO32 (aq) I(aq) +3SO42 (aq) (b) IO3(aq)+5I(aq)+6H+(aq) 3I2 (aq) +3H2O (liq) (c) I2 (aq) +starch(aq)I2-starch complex (BLUE!) In this reaction, iodate (IO3)and sulfite (SO32) react in (a) to form iodide (I)and sulfate (SO42). The sulfite will be the limiting reactant. Once the sulfite is used up, excess iodate will react with iodide (b) to form molecular iodine, resulting in a color change. The timing of the color change is so sharp and dependable that the reaction is referred to as a "clock" reaction. A clock reaction is any reaction with a sudden observable change occurs after a specific period of time. Since iodine itself is a faint yellow-brown color, starch is added as an indicator to make the color change more dramatic. 4. Write an experimental rate lavis for the teacong you stadied icday, after mouncievg off your reaction onders in the neures integer (orhalfinkeger). Post-lab Questions: 1. Identify all reactants, products and intermediates in the reaction you studied today, Recall that reactants are consumed in the overall reaction, products ate produced in the overall reaction, intermediates are produced in an carly step and then consumed in a later step, and catalysts are consumed in an early step and then produced in a later step. RCTS - 403,3SO32111 PDTS - 3SO4, 31+2012+L2 INTS - 2. By comparing the relative rates of reactions $1&2, determine the reaction order in [SO32]. Show your calculations, and show three significant figures in your final answer. 3. By comparing the relative rates of reactions \#1 \& 3, determine the reaction order in [IO3]. Show your calculations, and show three significant figures in your final answer. Write an experimental rate law for the reaction you studied today, after rounding off your reaction orders to the nearest integer (or half-integer)