Answered step by step
Verified Expert Solution
Question
1 Approved Answer
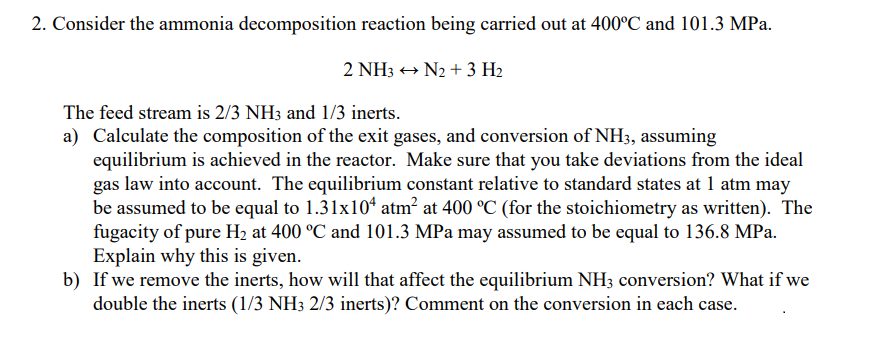
Please give an explanation with the answers, not just see work. Thanks! Consider the ammonia decomposition reaction being carried out at 400C and 101.3MPa. 2NH3N2+3H2
Please give an explanation with the answers, not just see work. Thanks!
Consider the ammonia decomposition reaction being carried out at 400C and 101.3MPa. 2NH3N2+3H2 The feed stream is 2/3NH3 and 1/3 inerts. a) Calculate the composition of the exit gases, and conversion of NH3, assuming equilibrium is achieved in the reactor. Make sure that you take deviations from the ideal gas law into account. The equilibrium constant relative to standard states at 1atm may be assumed to be equal to 1.31104atm2 at 400C (for the stoichiometry as written). The fugacity of pure H2 at 400C and 101.3MPa may assumed to be equal to 136.8MPa. Explain why this is given. b) If we remove the inerts, how will that affect the equilibrium NH3 conversion? What if we double the inerts ( 1/3NH32/3 inerts)? Comment on the conversion in each caseStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


