Question
Item 3 Part A How does the de Broglie wavelength of an electron change if its momentum increases? The de Broglie wavelength of the
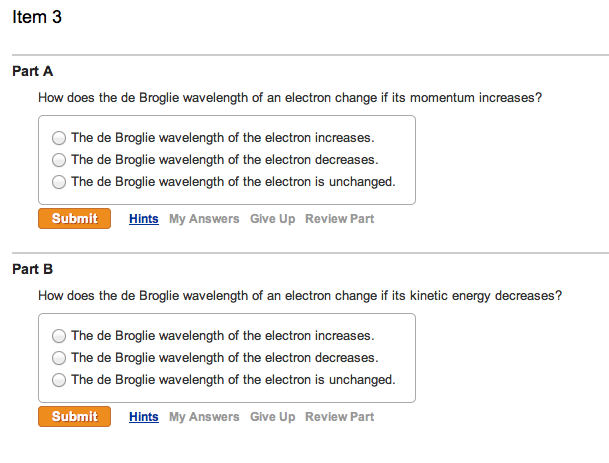
Item 3 Part A How does the de Broglie wavelength of an electron change if its momentum increases? The de Broglie wavelength of the electron increases. The de Broglie wavelength of the electron decreases. The de Broglie wavelength of the electron is unchanged. Submit Hints My Answers Give Up Review Part Part B How does the de Broglie wavelength of an electron change if its kinetic energy decreases? The de Broglie wavelength of the electron increases. The de Broglie wavelength of the electron decreases. The de Broglie wavelength of the electron is unchanged. Submit Hints My Answers Give Up Review Part
Step by Step Solution
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
Fall de Broglie Wavelength is defined as h d where h 32 E h is the plancks constant and ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: James S. Walker
5th edition
978-0133498493, 9780321909107, 133498492, 0321909100, 978-0321976444
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



