Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help!! 2. What is the molecular weight of N-ethylsaccharin and Q-ethylsaccharin or N-2-propylsaccharin and Q-2-propylsaccharin (use the correct products for the reaction that you
please help!! 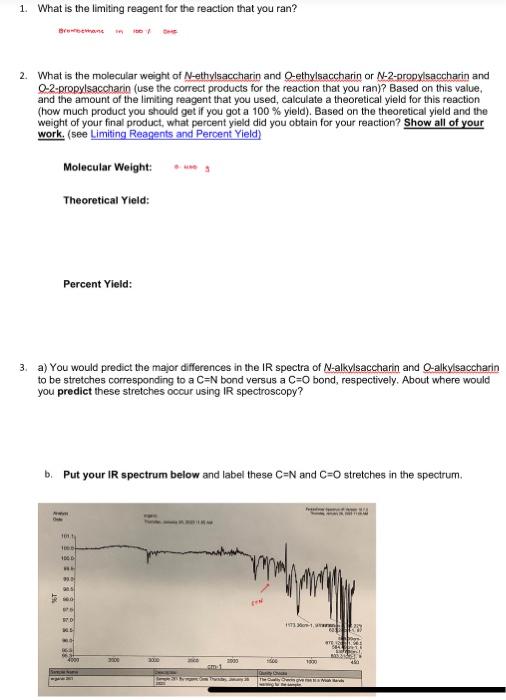
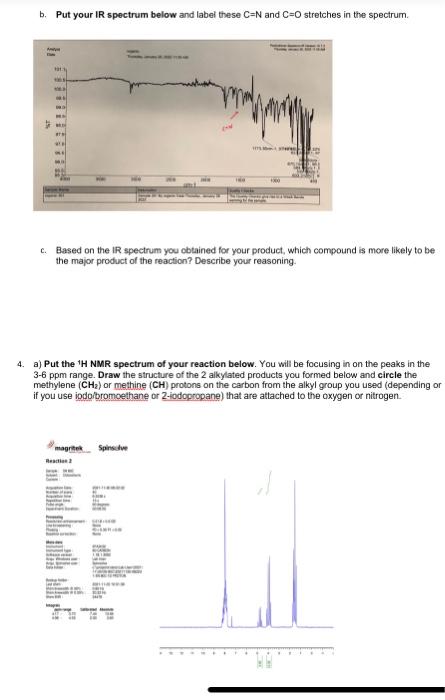


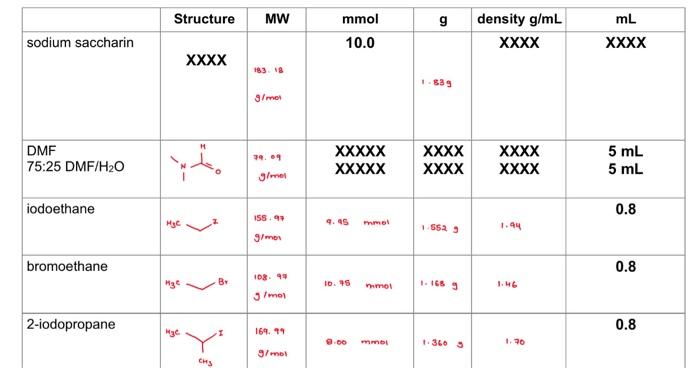
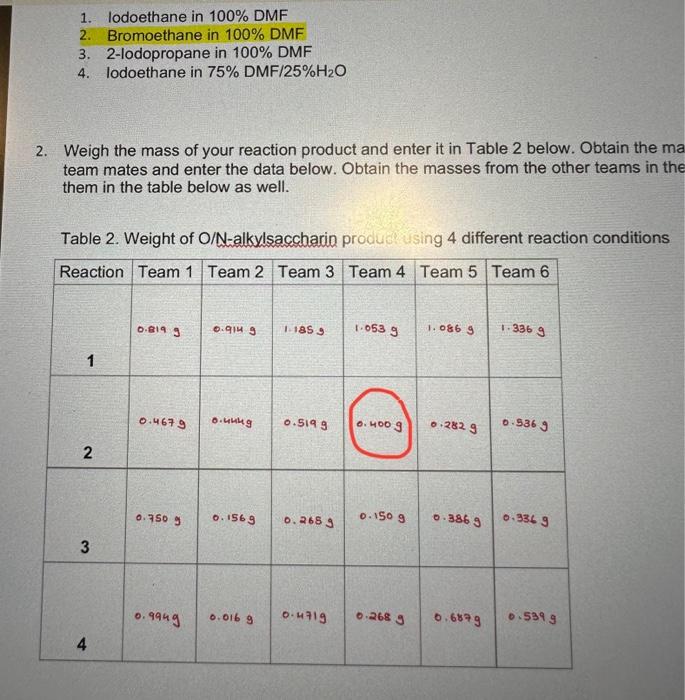
2. What is the molecular weight of N-ethylsaccharin and Q-ethylsaccharin or N-2-propylsaccharin and Q-2-propylsaccharin (use the correct products for the reaction that you ran)? Based on this value, and the amount of the limiting reagent that you used, calculate a theoretical yield for this reaction (how much product you should get if you got a 100% yield). Based on the theoretical yield and the weight of your final oroduct. what percent vield did you obtain for your reaction? Show all of your work. (see! Molecular Weight: s w s Theoretical Yield: Percent Yield: 3. a) You would predict the major differences in the IR spectra of N-alkylsaccharin and Q-alkylsaccharin to be stretches corresponding to a C=N bond versus a C=O bond, respectively. About where would you predict these stretches occur using R spectroscopy? b. Put your R spectrum below and label these C=N and C=O stretches in the spectrum. b. Put your IR spectrum below and label these C=N and C=O stretches in the spectrum. c. Based on the IR spectrum you obtained for your product, which compound is more likely to be the major product of the reaction? Describe your reasoning. 4. a) Put the 1H NMR spectrum of your reaction below. You will be focusing in on the peaks in the 3-6 ppm range. Draw the structure of the 2 alkylated products you formed below and circle the methylene (CH2) or methine (CH) protons on the carbon from the alkyl group you used (depending or if you use iodaibromoethane or 2-fodopropane) that are atlached to the oxygen or nitrogen. b. These methylene or methine protons next to the oxygen in the O-alkylated product will show up at a different position from those in the N-alkylated product. An oxygen atom has a stronger deshielding effect on nearby protons than a nitrogen atom. With this in mind, which of the 2 signals in the 3-6 ppm range correspond to the O-alkyl group and which is the N-alkyl group? c. Rationalize the splitting of the above signals for the methylene or methine protons that you observe in your NMR spectrum based on the number of neighboring protons. d. The integration areas for each of the 2 signals is below those signals in the NMR spectrum. Based on these integration values, determine the percentages of N-alkylsaceharin and O-alkylsaccharin in your product. Show you work. Provide these percentages in Table 3 below. e. Look at the NMR data for the other 3 reactions that your team mates ran and calculate the percentages of N-alkylsaccharin and Q-alkylsaccharin in these reactions as well. Provide these percentages in Table 3 below. Table 3. Percentage of N-alkylsaccharin and Q-alkvlsacchario in the substitution reactions f. Based on the percentages in Table 3, briefly (1-2 sentences) rationalize the difference in the percentages of N-alkylsaccharin and Q-alkylsaccharin between Reactions 1 and 3 5. Determine the average weight for each reaction condition and add it to Table 2 below. Table 2. Weight of O/N-alkylsacchario product using the 4 different reaction conditions 6. Based on the values of the average weight in Table 2 , briefly (1-2 sentences) rationalize the following: a. The difference in weights of Reactions 1 and 2 b. The difference in weights of Reactions 1 and 3 c. The difference in weights of Reactions 1 and 4 1. lodoethane in 100% DMF 2. Bromoethane in 100% DMF 3. 2-lodopropane in 100% DMF 4. lodoethane in 75%DMF/25%H2O Weigh the mass of your reaction product and enter it in Table 2 below. Obtain the me team mates and enter the data below. Obtain the masses from the other teams in thi them in the table below as well. Table 2. Weight of O/N-alkylsacchario produstusing 4 different reaction conditions 





Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


