PLEASE HELP 8,9,10

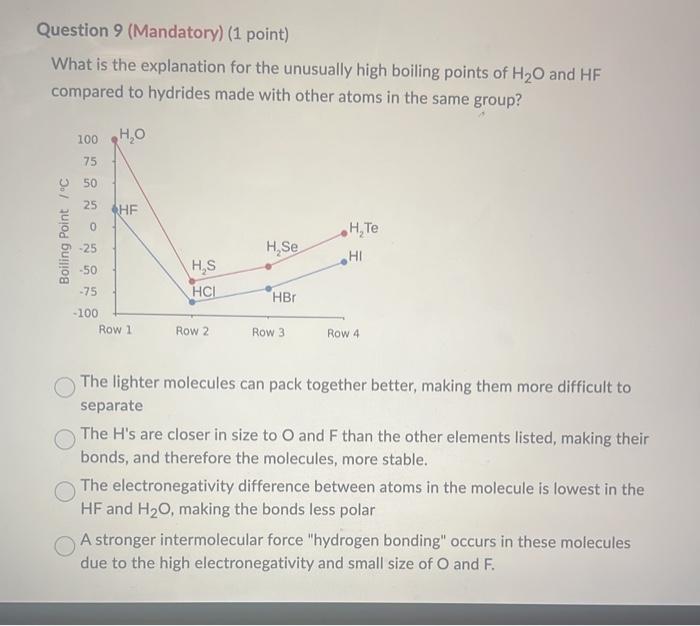
The gonerio structural formula for a 1-aligy.3-methylimidazolumin cation is where R is a CH2(CH2),CH3 alkyl group. The melting points of the salts. that form between the 1 -allyli-methylimidazolium cation and the PF6anion are as follows: R=CH2CH3(mp=60C)R=CH2CH2CH3(mp=40C)R=CH2CH2CH2CH3(mp=10C),andR=CH2CH2CH2CH2CH2CH3(m.p.=61C) Why does the melting point decrease as the length of alkyl group inereases? The longer the alkyl side chain, the greater the molecular weight of the compound. As a result, the larger molecules will have greater polarizability, and their melting points will increase in accordance with the increased molecular weights. The longer the alkyl side chain, the more irregular the shape of the cation and, as a result, the cation is more difficult to pack into a solid. The longer the alkyl side chain of the cation, the faster it rotates in any direction and, as result, the cation is more difficult to pack into solid. The longer the alkyl side chain of the cation, the greater the number of intermolecular attractions. This will make it more difficult for the molecule to pack together and solidify. The longer the alkyl side chain of the cation, the less delocalized the positive charge. As result, the cation is more difficult to pack into solid bacause of the high charge density What is the explanation for the unusually high boiling points of H2O and HF compared to hydrides made with other atoms in the same group? The lighter molecules can pack together better, making them more difficult to separate The H's are closer in size to O and F than the other elements listed, making their bonds, and therefore the molecules, more stable. The electronegativity difference between atoms in the molecule is lowest in the HF and H2O, making the bonds less polar A stronger intermolecular force "hydrogen bonding" occurs in these molecules due to the high electronegativity and small size of O and F. For the next three questions, refer to the chart below. The boiling points, surface tensions, and viscosities of water and several alcohols are as follows: For ethanol, propanol, and n-butanol the boiling points, surface tensions, and viscosities all increase in that order. What is the reason for this increase? The strength of the hydrogen bonding increases as you go from ethanol to propanol to n butanol. The type of dominant intermolecular force changes to stronger and stronger ones as you go from ethanol to propanol to n-butanol. The kinetic energy of the molecules increases as you go from ethanol to propanol, to n-butanol. The strength of the dispersion forces increases as you go from ethanol to propanol, to n-butanol