Answered step by step
Verified Expert Solution
Question
1 Approved Answer
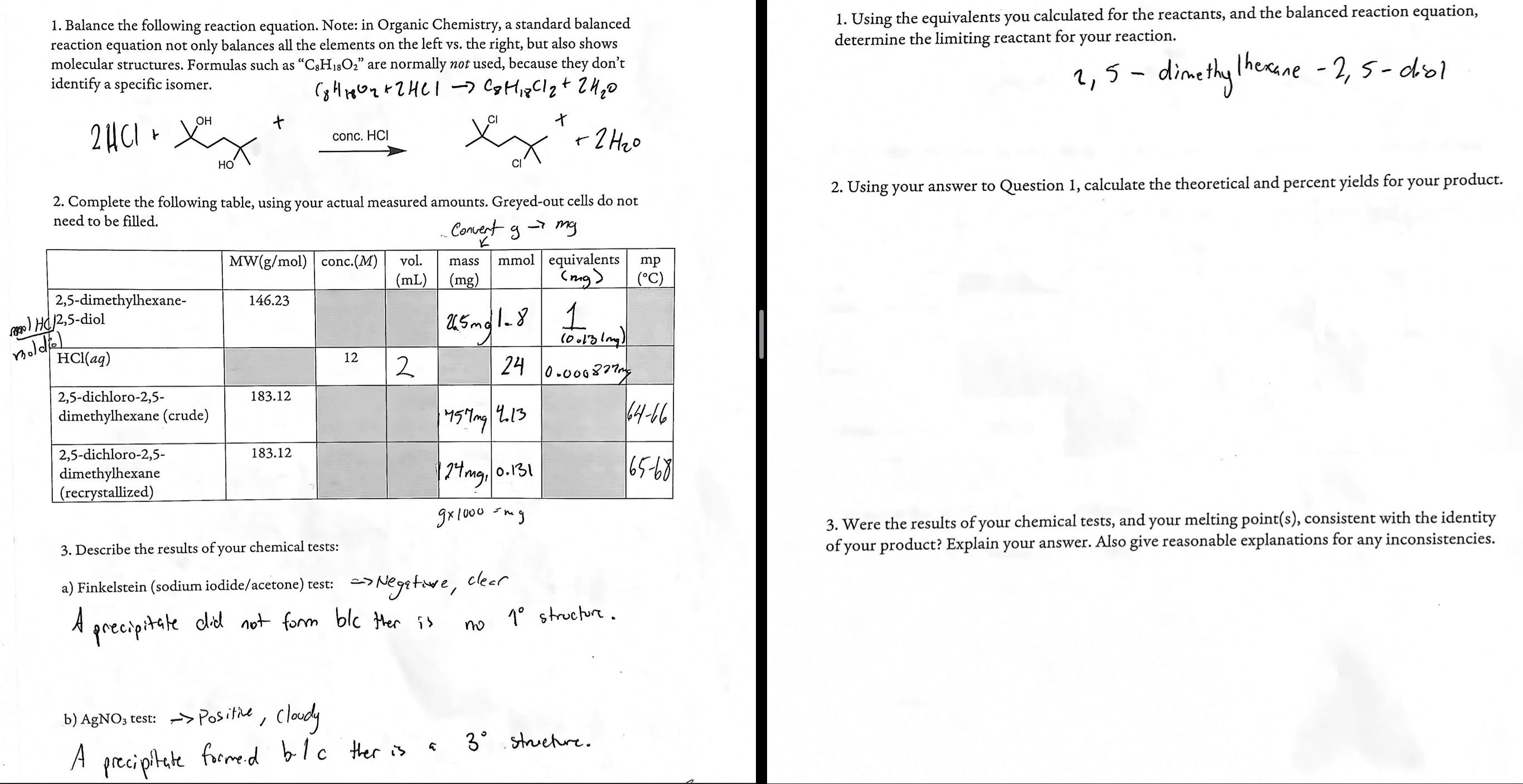
Please help answer the following! Will rate! SEE INFORMATION ON THE LAB BELOW! Background Lab information: A . Add 0 . 2 5 g of
Please help answer the following! Will rate! SEE INFORMATION ON THE LAB BELOW!
Background Lab information:
A Add g of dimethylhexanediol to mL vial. Dispense mL of concentrated hydrochloric acid to the vial. Cap the vial.
B Using the thermocouple attachment, heat mL beaker of water to degrees Celcius. While it heats collect the crude product by vacum filtration. Obtain a mass of the product. Recrystallize the product from methanol. Obtain the final mass of the product and its melting point. See charr for results
C Sulver Nitrate test: Transfer mg of the product to a small test tube, and dissolve it in a minimum amount of ethanol. Add mL of the supplied ethanolic silver nitrate solution to the test tube, and shake. Formation of a preciipitate within minutes is consistent with the presence of a tertiary alykl halide.
D Finkelstein test: Transfer mg of your product to a a test tube, and dissolve it in a minimum amount of acetone. Add mL of the supplied solution of NaI in acetone to the test tube, and shake. Formation of a precipitate within minutes is consitent with the presence of a prinary alkyl halide.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


