PLEASE HELP ASAP. SOLVE PART A USING MATLAB AND INCLUDE SCRIPT IN SOLUTION
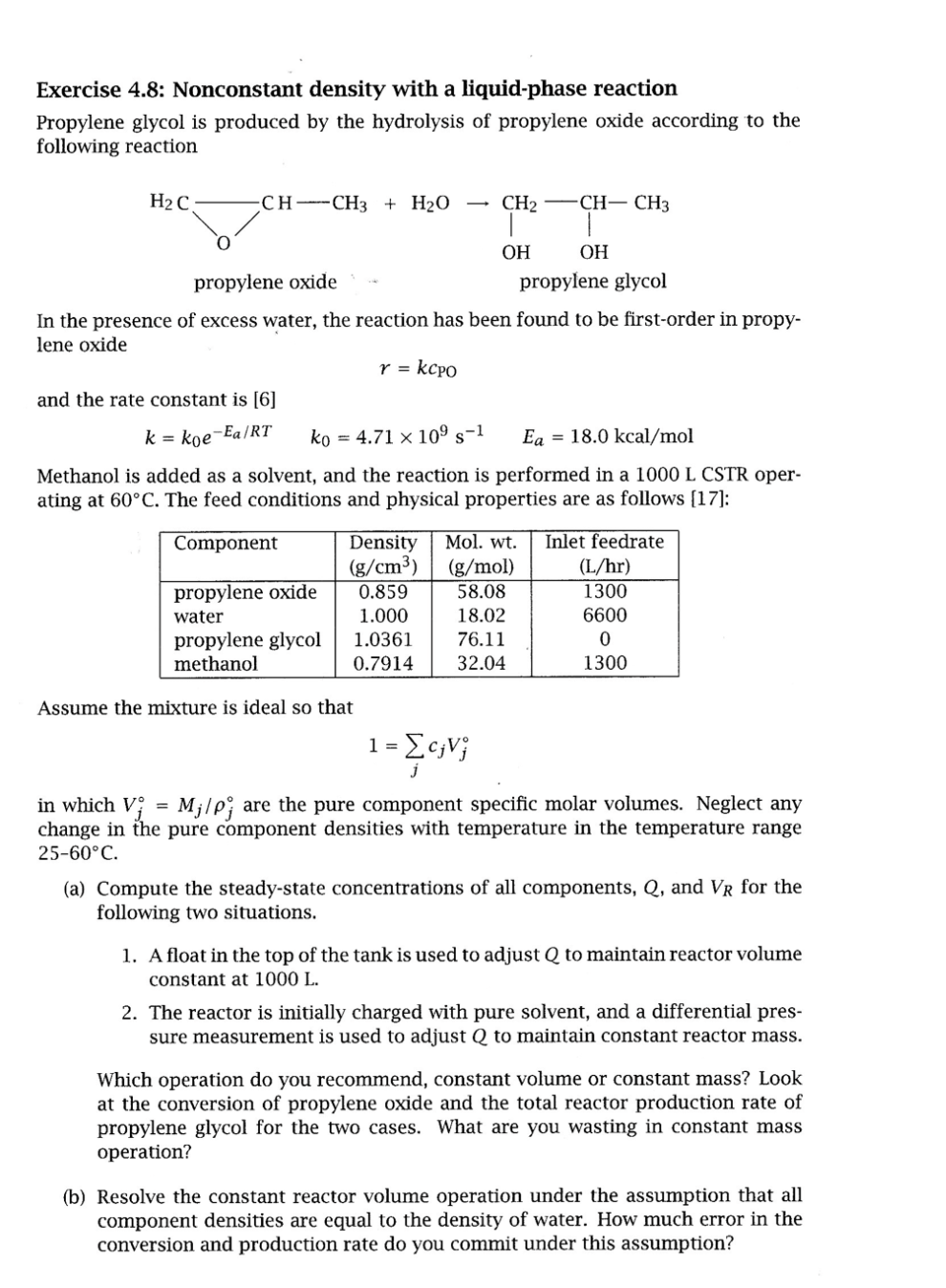
Exercise 4.8: Nonconstant density with a liquid-phase reaction Propylene glycol is produced by the hydrolysis of propylene oxide according to the following reaction In the presence of excess water, the reaction has been found to be first-order in propylene oxide r=kcPO and the rate constant is [6] k=k0eEa/RTk0=4.71109s1Ea=18.0kcal/mol Methanol is added as a solvent, and the reaction is performed in a 1000 L CSTR operating at 60C. The feed conditions and physical properties are as follows [17]: Assume the mixture is ideal so that 1=jcjVj in which Vj=Mj/j are the pure component specific molar volumes. Neglect any change in the pure component densities with temperature in the temperature range 2560C (a) Compute the steady-state concentrations of all components, Q, and VR for the following two situations. 1. A float in the top of the tank is used to adjust Q to maintain reactor volume constant at 1000L. 2. The reactor is initially charged with pure solvent, and a differential pressure measurement is used to adjust Q to maintain constant reactor mass. Which operation do you recommend, constant volume or constant mass? Look at the conversion of propylene oxide and the total reactor production rate of propylene glycol for the two cases. What are you wasting in constant mass operation? (b) Resolve the constant reactor volume operation under the assumption that all component densities are equal to the density of water. How much error in the conversion and production rate do you commit under this assumption? Exercise 4.8: Nonconstant density with a liquid-phase reaction Propylene glycol is produced by the hydrolysis of propylene oxide according to the following reaction In the presence of excess water, the reaction has been found to be first-order in propylene oxide r=kcPO and the rate constant is [6] k=k0eEa/RTk0=4.71109s1Ea=18.0kcal/mol Methanol is added as a solvent, and the reaction is performed in a 1000 L CSTR operating at 60C. The feed conditions and physical properties are as follows [17]: Assume the mixture is ideal so that 1=jcjVj in which Vj=Mj/j are the pure component specific molar volumes. Neglect any change in the pure component densities with temperature in the temperature range 2560C (a) Compute the steady-state concentrations of all components, Q, and VR for the following two situations. 1. A float in the top of the tank is used to adjust Q to maintain reactor volume constant at 1000L. 2. The reactor is initially charged with pure solvent, and a differential pressure measurement is used to adjust Q to maintain constant reactor mass. Which operation do you recommend, constant volume or constant mass? Look at the conversion of propylene oxide and the total reactor production rate of propylene glycol for the two cases. What are you wasting in constant mass operation? (b) Resolve the constant reactor volume operation under the assumption that all component densities are equal to the density of water. How much error in the conversion and production rate do you commit under this assumption







