Question: please help Evaluating Specific Gravity Specific Gravity is the ratio of the density of a substance divided by the density of water. Since water has
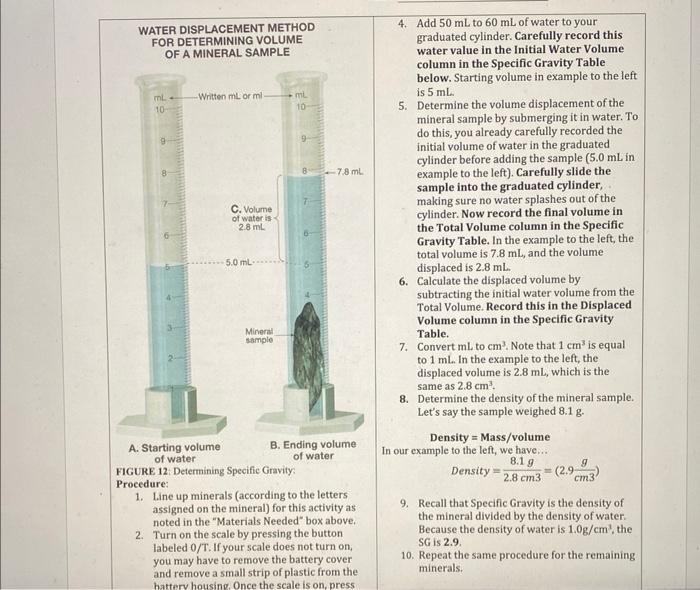

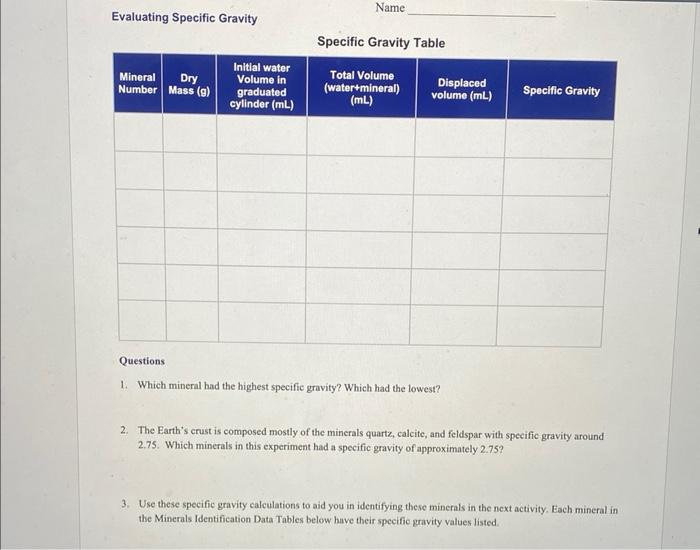
Evaluating Specific Gravity Specific Gravity is the ratio of the density of a substance divided by the density of water. Since water has a density of 1g/cm3, the units cancel out. Specific gravity (SG) is the same number as density, but without the units. The mineral quartz (density =2.65g/cm3 ) has a SG of 2.65. Hefting is an easy way to judge the specific gravity of one mineral relative to another. This is done by holding a mineral in one hand and holding an equal-sized different mineral in the other hand. Feel the difference in weight. The sample that feels heavier has the higher heft or specific gravity. A. A mineral sample weighs 27 grams and takes up 10.4cm3 of volume. What is the Specific Gravity of this mineral? Show your work. Name 4. Add 50mL to 60mL of water to your graduated cylinder. Carefully record this water value in the Initial Water Volume column in the Specific Gravity Table below. Starting volume in example to the left is 5mL. 5. Determine the volume displacement of the mineral sample by submerging it in water. To do this, you already carefully recorded the initial volume of water in the graduated cylinder before adding the sample (5.0mL in example to the left). Carefully slide the sample into the graduated cylinder. making sure no water splashes out of the WATER DISPLACEMENT METHOD 4. Add 50mL to 60mL of water to your FOR DETERMINING VOLUME graduated cylinder. Carefully record this water value in the Initial Water Volume column in the Specific Gravity Table below. Starting volume in example to the left is 5mL. 5. Determine the volume displacement of the mineral sample by submerging it in water. To do this, you already carefully recorded the initial volume of water in the graduated cylinder before adding the sample (5.0mL in example to the left). Carefully slide the sample into the graduated cylinder, making sure no water splashes out of the cylinder. Now record the final volume in the Total Volume column in the Specific Gravity Table. In the example to the left, the total volume is 7.8mL, and the volume displaced is 2.8mL. 6. Calculate the displaced volume by subtracting the initial water volume from the Total Volume. Record this in the Displaced Volume column in the Specific Gravity Table. 7. Convert mL to cm3. Note that 1cm3 is equal to 1mL. In the example to the left, the displaced volume is 2.8mL, which is the same as 2.8cm3. 8. Determine the density of the mineral sample. Let's say the sample weighed 8.1g. Density = Mass / volume In our example to the left, we have... FIGURE 12: Determining Specific Gravity: Procedure: 1. Line up minerals (according to the letters assigned on the mineral) for this activity as noted in the "Materials Needed" box above. 2. Turn on the scale by pressing the button labeled 0/T. If your scale does not turn on, you may have to remove the battery cover and remove a small strip of plastic from the 9. Recall that Specific Gravity is the density of the mineral divided by the density of water. Because the density of water is 1.0g/cm3, the SG is 2.9. 10. Repeat the same procedure for the remaining hattery housing, Once the scale is on, press minerals. to 1mL. In the example to the left, the displaced volume is 2.8mL, which is the same as 2.8cm3. 8. Determine the density of the mineral sample. Let's say the sample weighed 8.1g. A. Starting volume B. Ending volume Density = Mass / volume of water of water FIGURE 12: Determining Specific Gravity: Procedure: 1. Line up minerals (according to the letters assigned on the mineral) for this activity as 9. Recall that Specific Gravity is the density of noted in the "Materials Needed" box above. the mineral divided by the density of water. 2. Turn on the scale by pressing the button Because the density of water is 1.0g/cm3, the labeled 0 /T. If your scale does not turn on, SG is 2.9. you may have to remove the battery cover 10. Repeat the same procedure for the remaining and remove a small strip of plastic from the minerals. battery housing. Once the scale is on, press the 0/T button a second time to zero the scale. Make sure that the units are in grams (g). If not, press the M button until the units displayed are in grams. 3. Weigh the sample in grams on a mass balance. Record the dry weight of Mineral A the Specific Gravity Table. Evaluating Specific Gravity Name Specific Gravity Table Questions 1. Which mineral had the highest specific gravity? Which had the lowest? 2. The Earth's crust is composed mosily of the minerals quartz, calcite, and feldspar with specific gravity around 2.75. Which minerals in this experiment had a specific gravity of approximately 2.75 ? 3. Use these specific gravity calculations to aid you in identifying these minerals in the next activity. Each mineral in the Minerals Identification Data Tables below have their specific gravity values listed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


