Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help!! I have no idea where to start with this. From what I understand, I need to figure out what intermediate is formed, and
Please help!! I have no idea where to start with this. From what I understand, I need to figure out what intermediate is formed, and through which mechanism it is made. Added are everything you should need, the experiment procedure, the spectras, and the notes and observations of the lab.


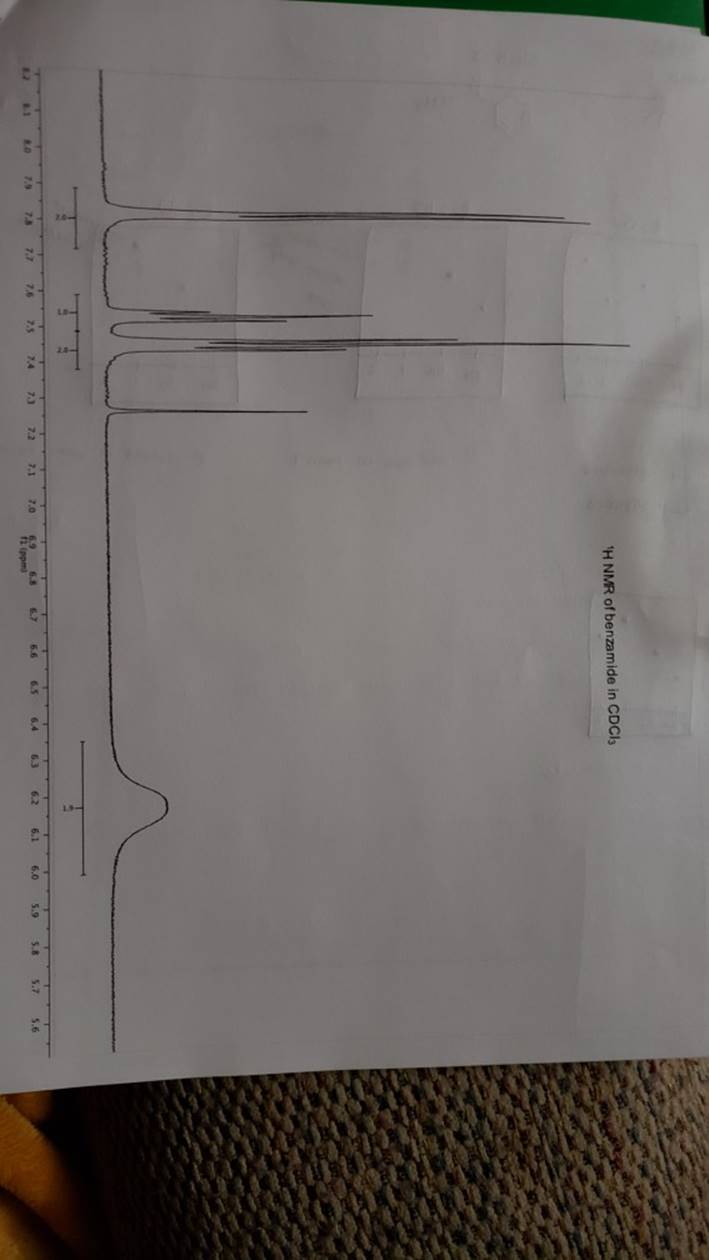
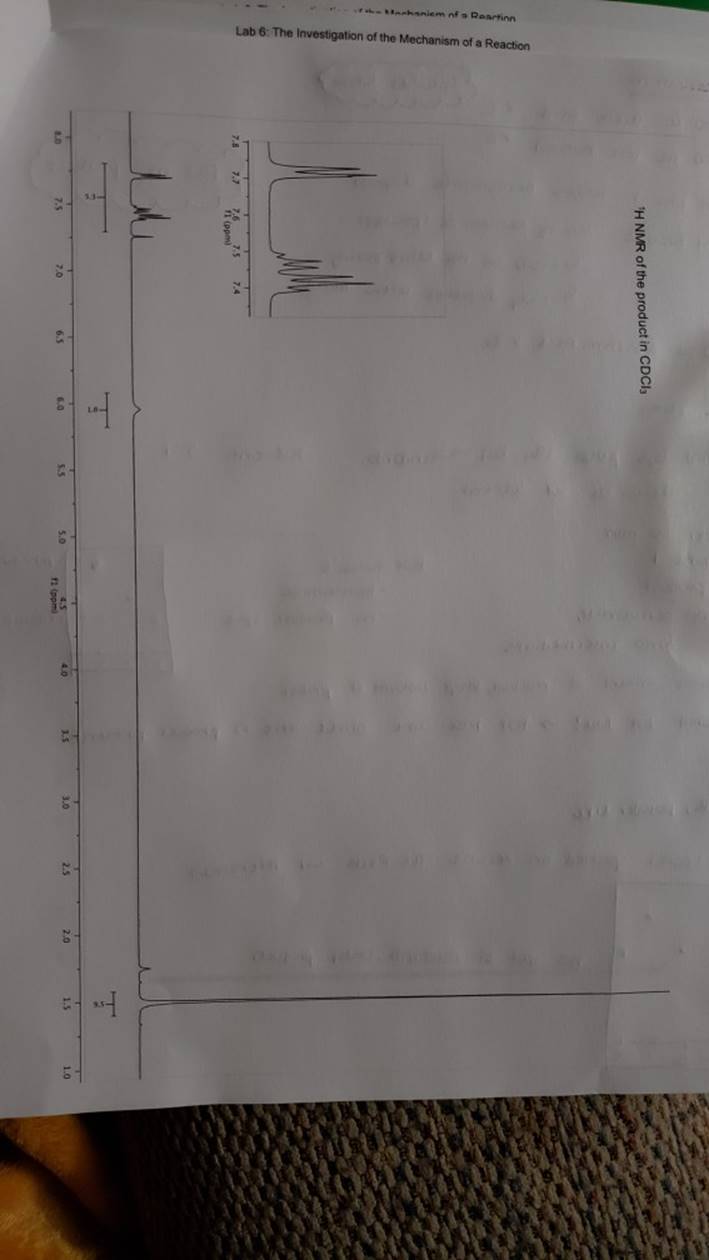
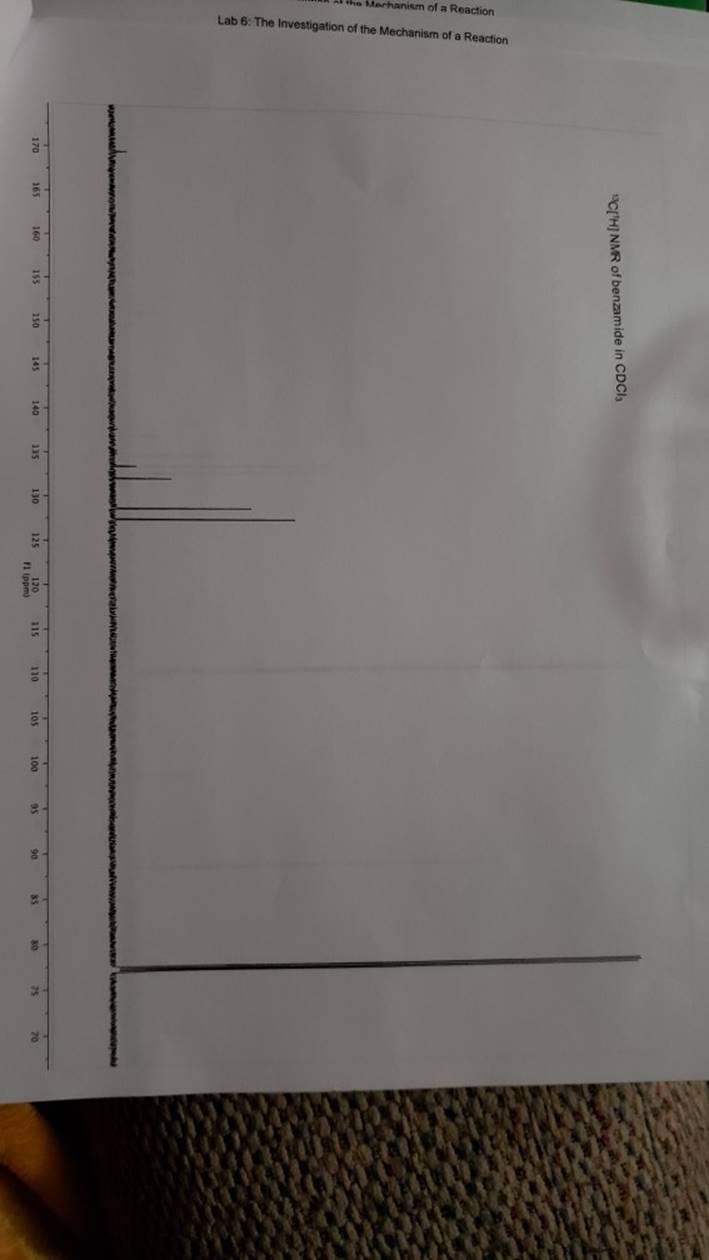
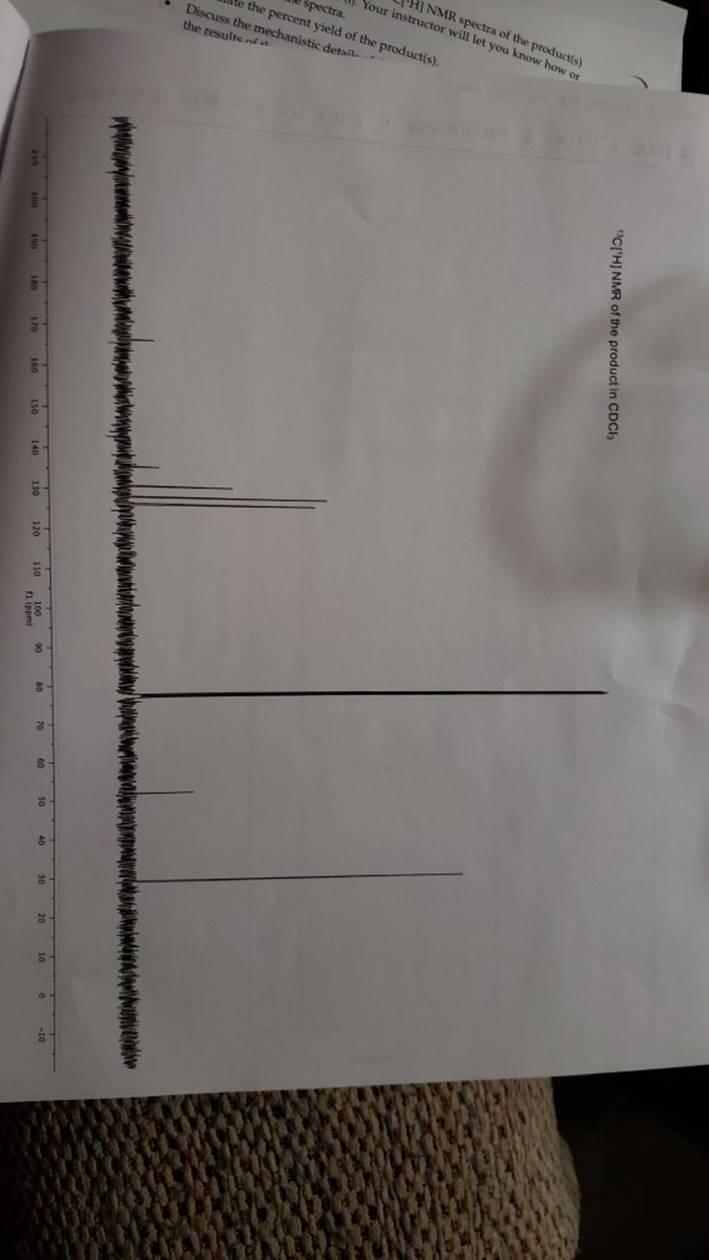
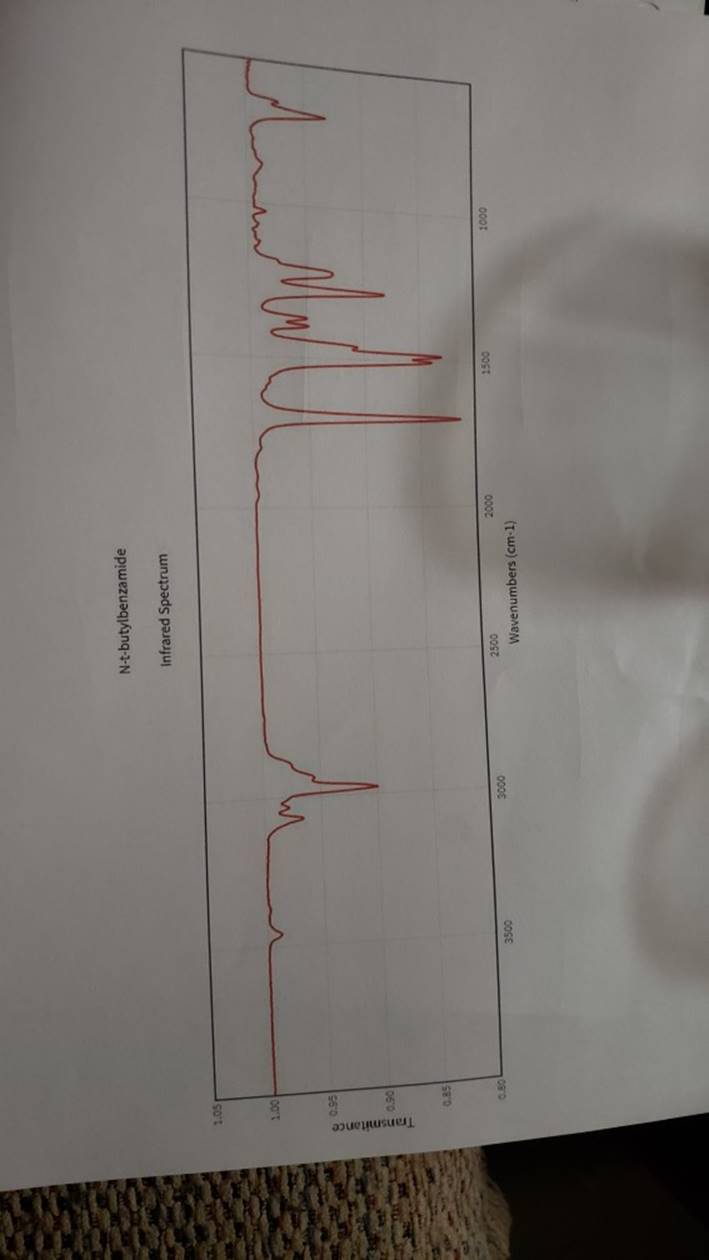
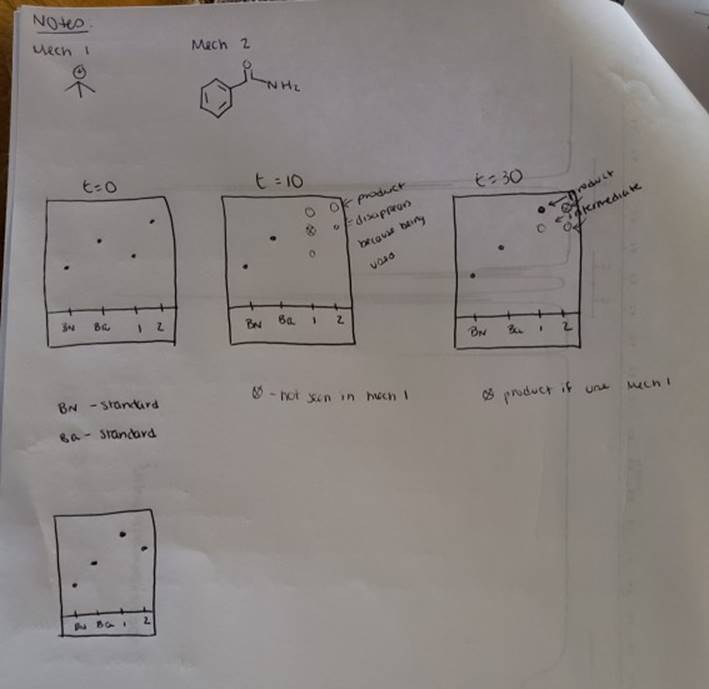

megd on of the Mechanism of a Reaction Lab 6: The Investigation of the Mechanism of a Reaction mechanisms are well understood and based on all of the available experimen- tal data. However, some may not have been verified by experiment and would need to be tested to determine if they are feasible pathways for the reaction. In this experiment, you will investigate the possible presence of an intermediate in the reaction of a nitrile and a tertiary alcohol in aqueous sulfuric acid. When benzonitrile is reacted with tert-butyl alcohol in aqueous sulfuric acid, the product is N-tert-butylbenzamide (Equation 1). HSO -CEN HO (1) benzonitrile N-tert-butylbenzamide In one possible mechanism, benzamide is formed as an intermediate by acid hydrolysis of benzonitrile. The benzamide then reacts with the tert- butyl carbocation formed from tert-butyl alcohol and sulfuric acid. In an alternative mechanism, the tert-butyl carbocation adds to the benzonitrile to form a carbon-nitrogen bond, resulting in a nitrilium ion. Water then adds to this intermediate to give the final product. Background Reading: Review the acid-catalyzed hydrolysis of nitriles in your lecture text. Prelab Checklist: Pre-lab questions Include the structures and physical properties of the reactants and the solvents used in this experiment in your prelab write-up. Write at least two reasonable arrow-pushing mechanisms for the reaction. Experimental Procedure Wear your safety glasses at all times in the lab. In each of two reaction tubes/small test tubes, measure 0.7 ml glacial acetic acid and 0.2 mL 2-methyl-2-propanol (tert-butyl alcohol) and mix them well by stirring TECHNIQUE TIP The glass stirring rod displaces too much volume so it is preferable to use the stainless steel spatula for stirring these solutions SAFETY NOTE The stainless steel spatula has acid on it. Have a test tube of 10% aqueous NaHCO, available and place the spatula in this solution immediately after removing it from the reaction mixture. Care should also be taken with micropipettes used to sample the reaction solution for thin layer chromatography because these are also contam- inated with the acid solution. custom page 40
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


