Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help :) i will thumbs up Use the References to access important values if needed for this question. A 9.649 mol sample of nitrogen
please help :) i will thumbs up 
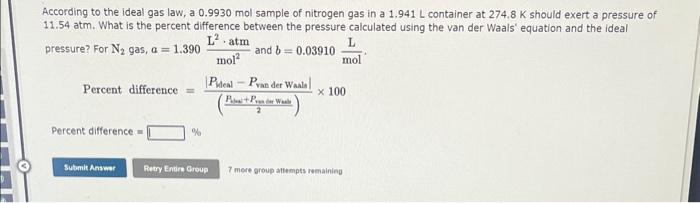
Use the References to access important values if needed for this question. A 9.649 mol sample of nitrogen gas is maintained in a 0.8438L container at 303.0K. What is the pressure in atm calculated using the van der Waals' equation for N2 gas under these conditions? For N2,a=1.390mol2L2atm and b=0.03910molL. Pressure = 7 more group attem@ts remaining According to the ideal gas law, a 0.9930mol sample of nitrogen gas in a 1.941L container at 274.8K should exert a pressure of 11.54atm. What is the percent difference between the pressure calculated using the van der Waals' equation and the ideal pressure? For N2 gas, a=1.390mol2L2atm and b=0.03910molL. Percentdifference=(2Pbut+Predrwat)PMealPvanderWaala100 Percent difference = 7 mere aroup attempts remaining 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


