Question
n-Butane is converted to isobutane in a continuous isomerization reactor that operates isothermally at 150 C. n-C4H10 (g) i-C4H10 (g) The feed to the
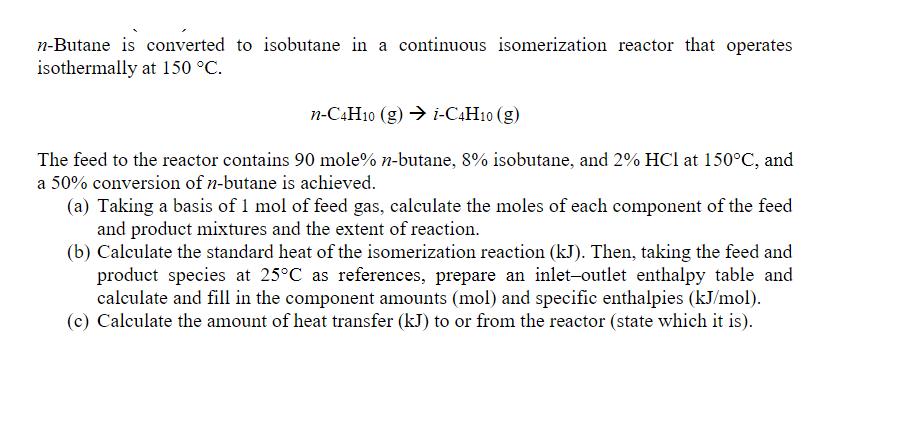
n-Butane is converted to isobutane in a continuous isomerization reactor that operates isothermally at 150 C. n-C4H10 (g) i-C4H10 (g) The feed to the reactor contains 90 mole% n-butane, 8% isobutane, and 2% HCl at 150C, and a 50% conversion of n-butane is achieved. (a) Taking a basis of 1 mol of feed gas, calculate the moles of each component of the feed and product mixtures and the extent of reaction. (b) Calculate the standard heat of the isomerization reaction (kJ). Then, taking the feed and product species at 25C as references, prepare an inlet-outlet enthalpy table and calculate and fill in the component amounts (mol) and specific enthalpies (kJ/mol). (c) Calculate the amount of heat transfer (kJ) to or from the reactor (state which it is).
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
I clea...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Separation process principles
Authors: J. D. Seader
2nd Edition
471464805, 978-0471464808
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



