Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help me answer all auestions not juat one! also please make clear where your wrote answers! i will like your work and thank you!
please help me answer all auestions not juat one! also please make clear where your wrote answers! i will like your work and thank you! 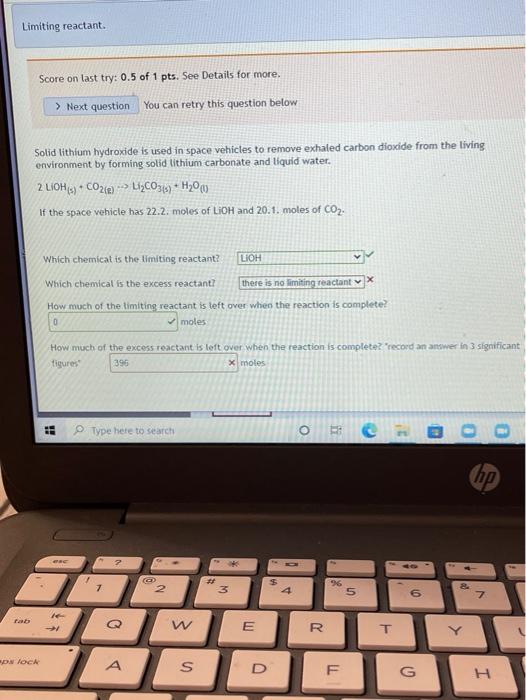
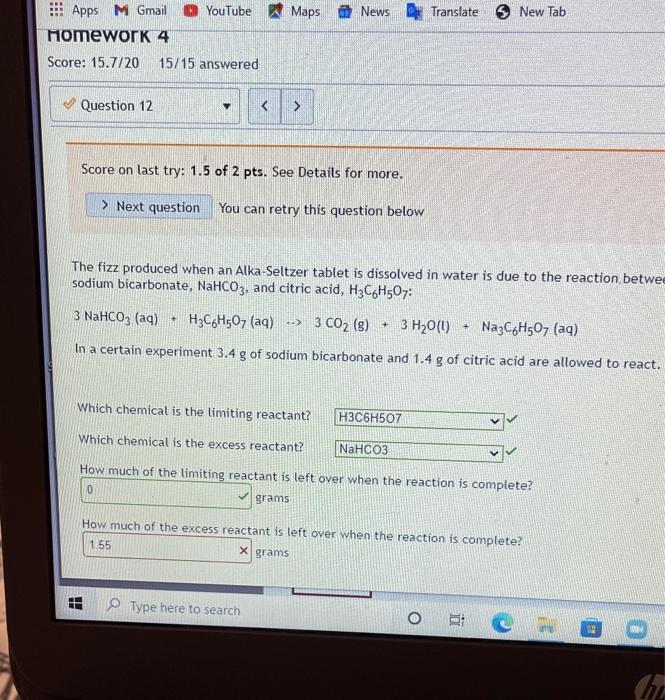
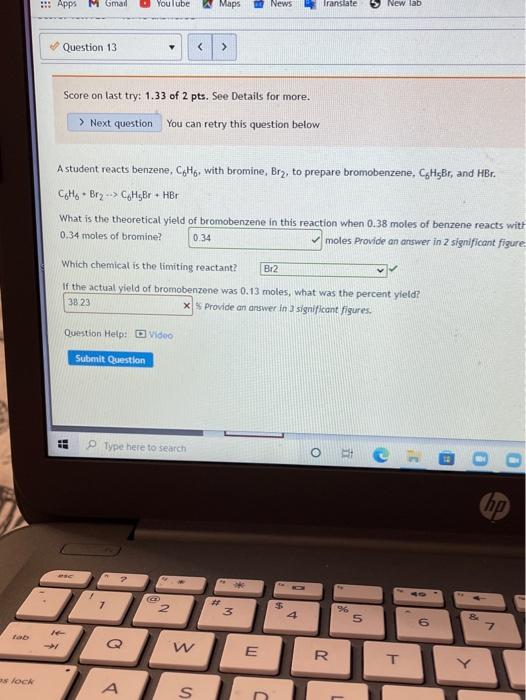
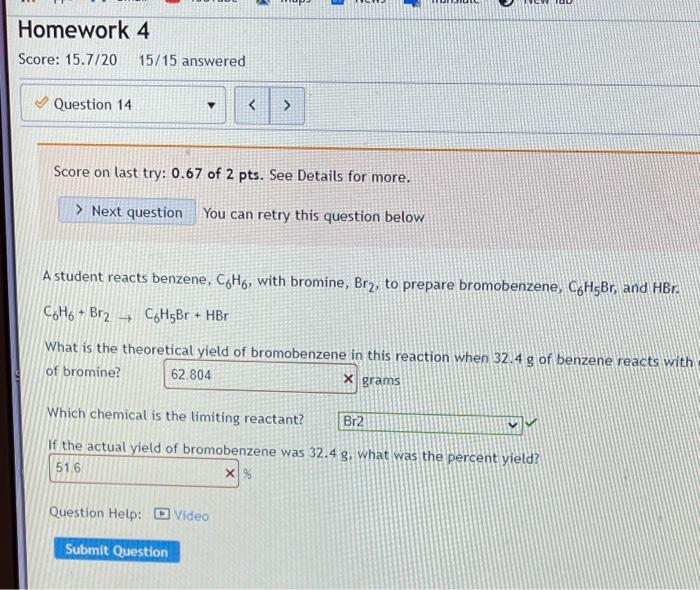
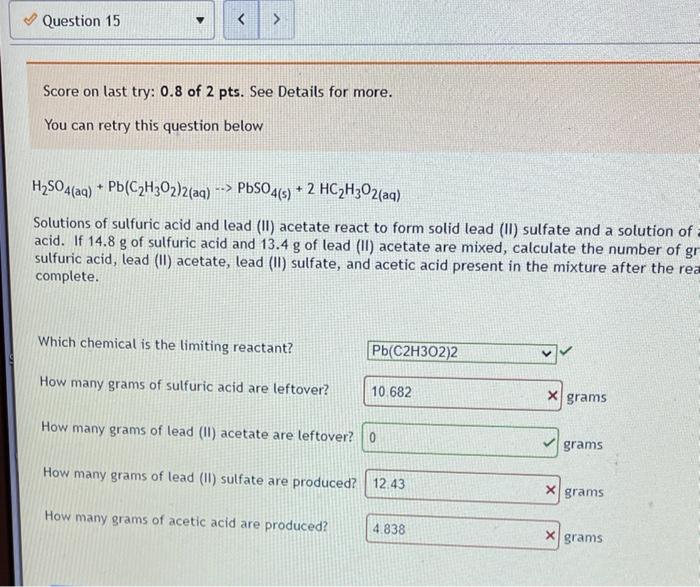
Limiting reactant. Score on last try: 0.5 of 1 pts. See Details for more. > Next question You can retry this question below Solid lithium hydroxide is used in space vehicles to remove exhaled carbon dioxide from the living environment by forming solid lithium carbonate and liquid water. 2 LIOH) + CO2) LI>C0319 + H20 If the space vehicle has 22.2. moles of LiOH and 20.1. moles of CO2, > Which chemical is the limiting reactant? LIOH Which chemical is the excess reactant? there is no limiting reactant How much of the timiting reactant is left over when the reaction is complete? 0 moles How much of the excess reactant is left over when the reaction is complete? "record an answer in 3 significant figures 396 x moles . 1 Type here to search o 1 @ D 0 hp 3 7 2 3 $ 4 96 5 6 Z N W E R 20 T Score on last try: 1.5 of 2 pts. See Details for more. > Next question You can retry this question below The fizz produced when an Alka-Seltzer tablet is dissolved in water is due to the reaction betwee sodium bicarbonate, NaHCO3, and citric acid, H3C6H507: 3 NaHCO3(aq) + H3C6H507 (aq) > 3 CO2 (s) + 3 H20(0) NazC6H507 (aq) In a certain experiment 3.4 g of sodium bicarbonate and 1.4 g of citric acid are allowed to react. Which chemical is the limiting reactant? H3C6H507 Which chemical is the excess reactant? NaHCO3 How much of the limiting reactant is left over when the reaction is complete? 0 grams How much of the excess reactant is left over when the reaction is complete? 1.55 X grams Type here to search o : C he !!! Apps M Gmal You lube Maps News Translate New lab Question 13 Score on last try: 1.33 of 2 pts. See Details for more. > Next question You can retry this question below Astudent reacts benzene, CoMo, with bromine, Bre, to prepare bromobenzene, CH Br, and HBr. CH. Br2 --> CHBr + Br What is the theoretical yield of bromobenzene in this reaction when 0.38 moles of benzene reacts with 0.34 moles of bromine? 0.34 moles Provide an answer in 2 significant figure Which chemical is the limiting reactant? Br2 If the actual yield of bromobenzene was 0.13 moles, what was the percent yield? 38 23 x S Provide an answer in 3 significant figures. Question Help: Video Submit Question !! o Type here to search o O E ce (hp 2 * 7 2 3 4 5 07 7 W E R. lock A A S n Homework 4 Score: 15.7/20 15/15 answered Question 14 v Score on last try: 0.67 of 2 pts. See Details for more. > Next question You can retry this question below -> A student reacts benzene, CoH6, with bromine, Brz, to prepare bromobenzene, C6H5Br. and HBr. CoHo - Br2 - CH5Br + HBr What is the theoretical yield of bromobenzene in this reaction when 32.4 g of benzene reacts with of bromine? 62.804 X grams Which chemical is the limiting reactant? Br2 If the actual yield of bromobenzene was 32.4 g, what was the percent yield? 51.6 X% Question Help: D Video Submit Question Question 15 PbSO4(s) + 2 HC2H302(aq) Solutions of sulfuric acid and lead (11) acetate react to form solid lead (II) sulfate and a solution of acid. If 14.8 g of sulfuric acid and 13.4 g of lead (II) acetate are mixed, calculate the number of gr sulfuric acid, lead (11) acetate, lead (II) sulfate, and acetic acid present in the mixture after the rea complete. Which chemical is the limiting reactant? Pb(C2H302)2 How many grams of sulfuric acid are leftover? 10.682 X grams How many grams of lead (11) acetate are leftover? O grams How many grams of lead (II) sulfate are produced? 12.43 x grams How many grams of acetic acid are produced? 4.838 x grams 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


