Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help me ask all the questions (four questions). I want the complete solution. Q1: the following table gives the results of enzyme-catalyzed reaction. The
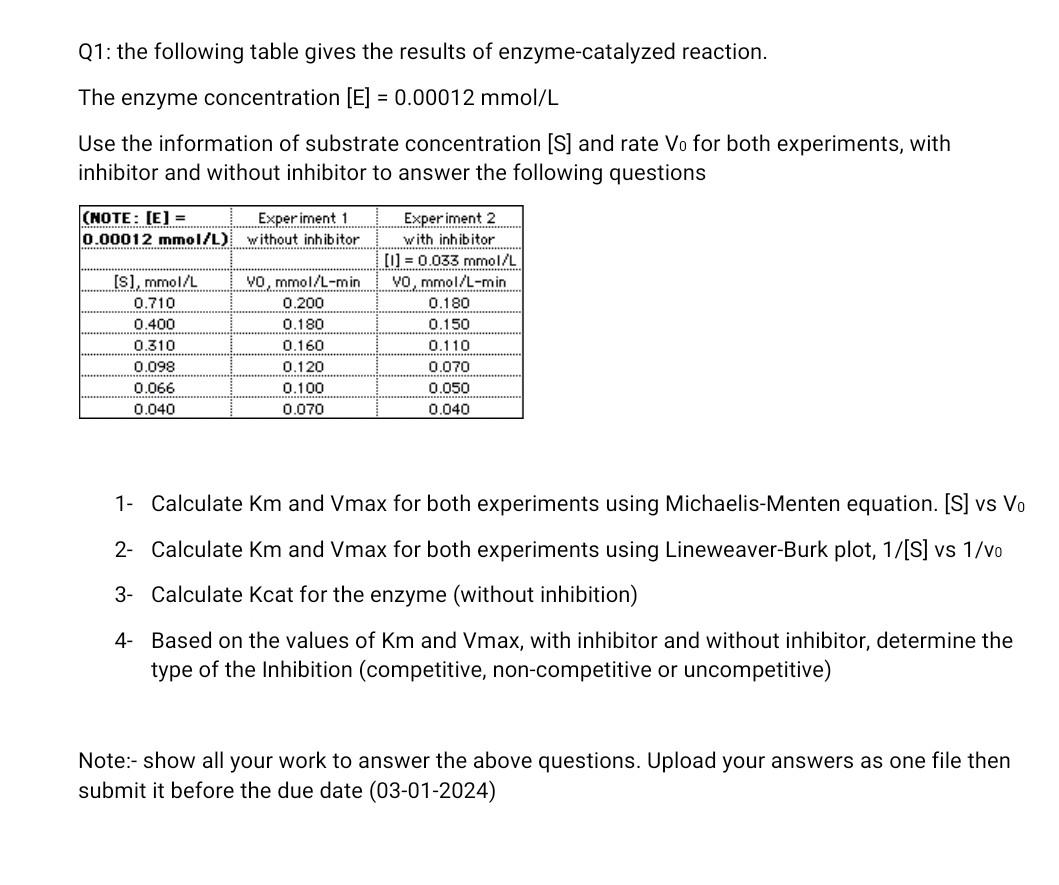
Please help me ask all the questions (four questions). I want the complete solution.
Q1: the following table gives the results of enzyme-catalyzed reaction. The enzyme concentration [E]=0.00012mmol/L Use the information of substrate concentration [S] and rate V0 for both experiments, with inhibitor and without inhibitor to answer the following questions 1- Calculate Km and Vmax for both experiments using Michaelis-Menten equation. [S] vs V0 2- Calculate Km and Vmax for both experiments using Lineweaver-Burk plot, 1/[S] vs 1/v0 3- Calculate Kcat for the enzyme (without inhibition) 4- Based on the values of Km and Vmax, with inhibitor and without inhibitor, determine the type of the Inhibition (competitive, non-competitive or uncompetitive) Note:- show all your work to answer the above questions. Upload your answers as one file then submit it before the due date (03-01-2024) Q1: the following table gives the results of enzyme-catalyzed reaction. The enzyme concentration [E]=0.00012mmol/L Use the information of substrate concentration [S] and rate V0 for both experiments, with inhibitor and without inhibitor to answer the following questions 1- Calculate Km and Vmax for both experiments using Michaelis-Menten equation. [S] vs V0 2- Calculate Km and Vmax for both experiments using Lineweaver-Burk plot, 1/[S] vs 1/v0 3- Calculate Kcat for the enzyme (without inhibition) 4- Based on the values of Km and Vmax, with inhibitor and without inhibitor, determine the type of the Inhibition (competitive, non-competitive or uncompetitive) Note:- show all your work to answer the above questions. Upload your answers as one file then submit it before the due date (03-01-2024)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


