Question
The heat capacity of hydrogen may be represented by Cpm = (1.554 + 0.00227) J K mol1 Calculate the change in entropy of the
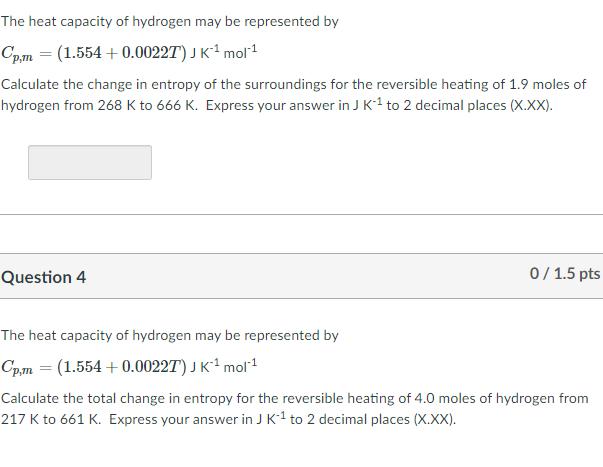
The heat capacity of hydrogen may be represented by Cpm = (1.554 + 0.00227) J K mol1 Calculate the change in entropy of the surroundings for the reversible heating of 1.9 moles of hydrogen from 268 K to 666 K. Express your answer in J K1 to 2 decimal places (X.XX). Question 4 0/ 1.5 pts The heat capacity of hydrogen may be represented by Cpm = (1.554 + 0.00227) JK mol Calculate the total change in entropy for the reversible heating of 4.0 moles of hydrogen from 217 K to 661 K. Express your answer in J K1 to 2 decimal places (X.XX).
Step by Step Solution
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
3 cp 1554 00022T Jk moli for reversible adiabatic process AS sys CP Ti 2 po...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Joseph M. Hornback
2nd Edition
9781133384847, 9780199270293, 534389511, 1133384846, 978-0534389512
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



