Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help me solve these two questions! if you solve both correctly i will give thumbs up. At a certain temperature, the equilibrium constant for
please help me solve these two questions! if you solve both correctly i will give thumbs up. 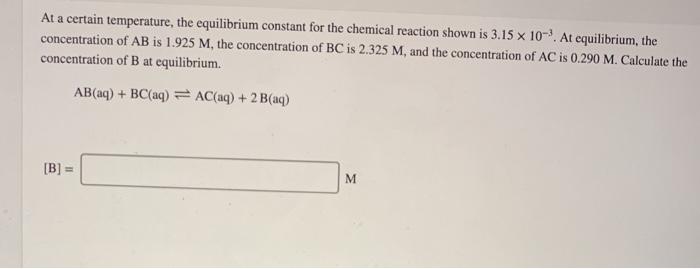
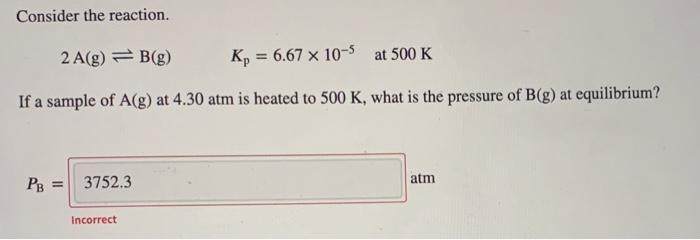
At a certain temperature, the equilibrium constant for the chemical reaction shown is 3.15 x 10-. At equilibrium, the concentration of AB is 1.925 M, the concentration of BC is 2.325 M, and the concentration of AC is 0.290 M. Calculate the concentration of B at equilibrium. AB(aq) + BC(aq) = AC(aq) + 2 B(aq) [B] = M Consider the reaction. 2 A(g) = B(g) Kp = = 6,67 x 10-5 at 500 K If a sample of A(g) at 4.30 atm is heated to 500 K, what is the pressure of B(g) at equilibrium? 3752.3 atm PB = Incorrect 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


