Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help! provide detailed explination L-Serine is a nonessential amino acid, but can be a useful constituent of a healthy diet and is often provided
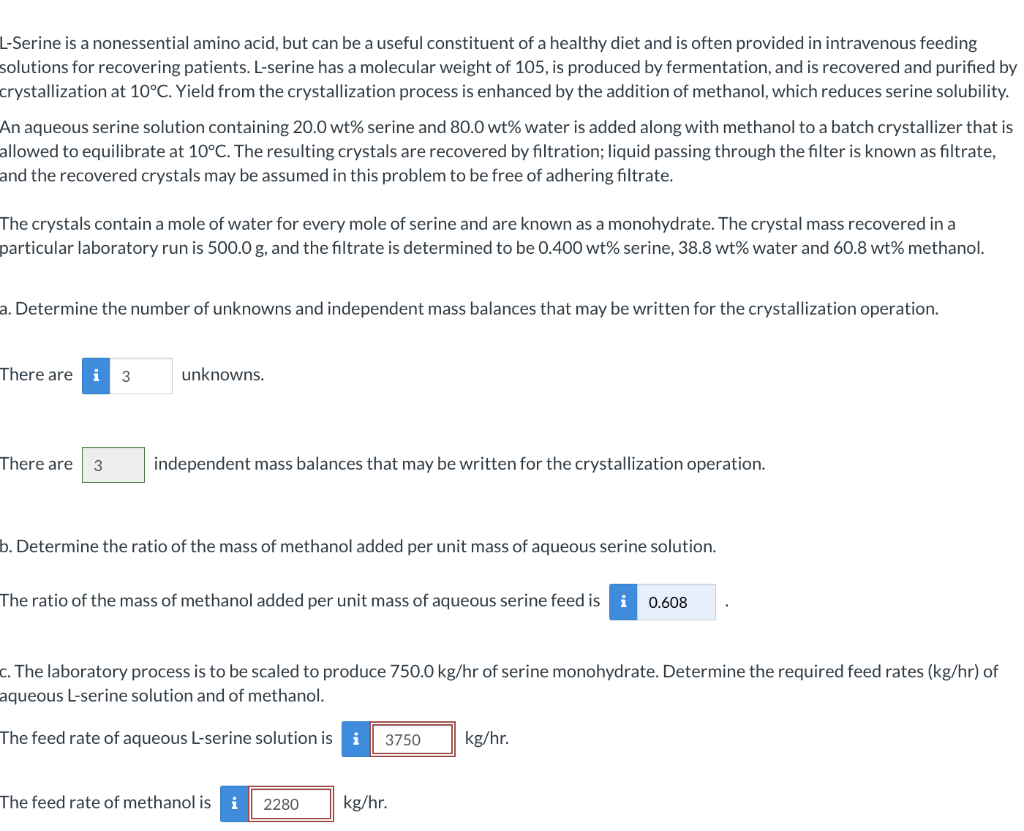
please help! provide detailed explination
L-Serine is a nonessential amino acid, but can be a useful constituent of a healthy diet and is often provided in intravenous feeding solutions for recovering patients. L-serine has a molecular weight of 105 , is produced by fermentation, and is recovered and purified by crystallization at 10C. Yield from the crystallization process is enhanced by the addition of methanol, which reduces serine solubility. An aqueous serine solution containing 20.0wt% serine and 80.0wt% water is added along with methanol to a batch crystallizer that is allowed to equilibrate at 10C. The resulting crystals are recovered by filtration; liquid passing through the filter is known as filtrate, and the recovered crystals may be assumed in this problem to be free of adhering filtrate. The crystals contain a mole of water for every mole of serine and are known as a monohydrate. The crystal mass recovered in a particular laboratory run is 500.0g, and the filtrate is determined to be 0.400wt% serine, 38.8wt% water and 60.8wt%methanol. a. Determine the number of unknowns and independent mass balances that may be written for the crystallization operation. There are unknowns. There are independent mass balances that may be written for the crystallization operation. b. Determine the ratio of the mass of methanol added per unit mass of aqueous serine solution. The ratio of the mass of methanol added per unit mass of aqueous serine feed is c. The laboratory process is to be scaled to produce 750.0kg/hr of serine monohydrate. Determine the required feed rates ( kg/hr ) of aqueous L-serine solution and of methanol. The feed rate of aqueous L-serine solution is kg/hr The feed rate of methanol is kg/hrStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


