Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help (Q2) In Haber's process, nitrogen and hydrogen are the reactants for the synthesis of ammonia. The operating conditions required in the reactor are
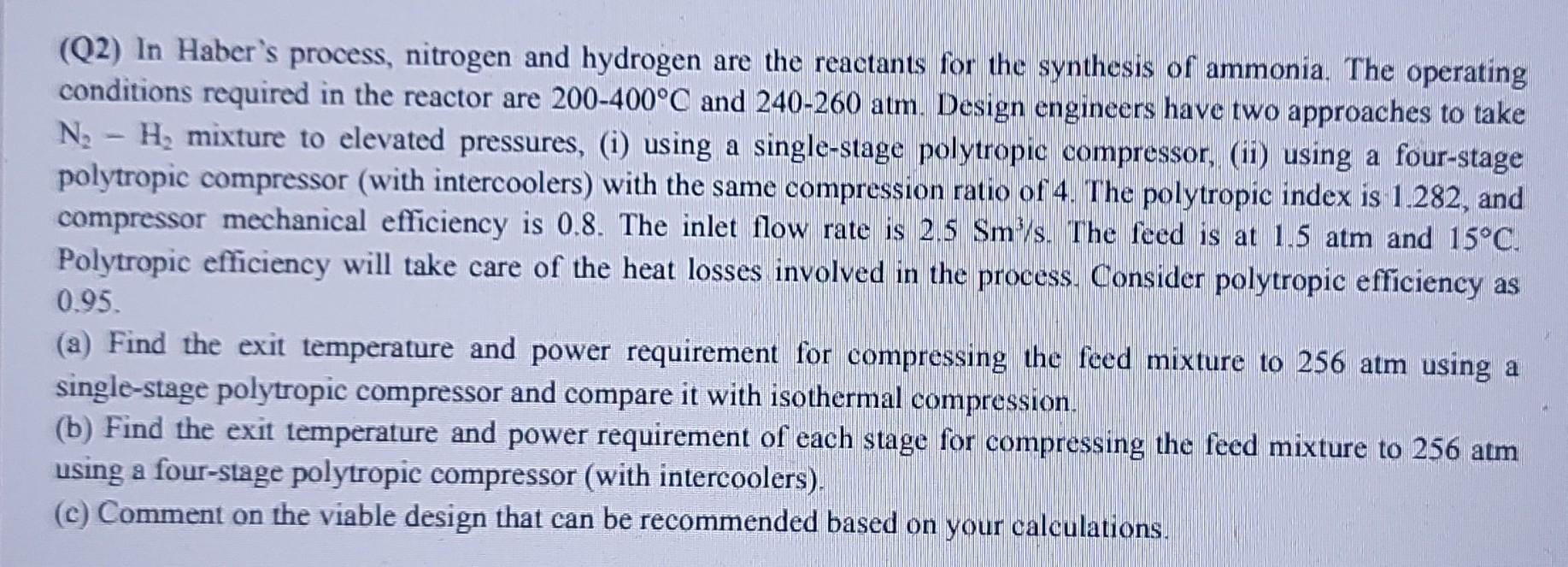
please help
(Q2) In Haber's process, nitrogen and hydrogen are the reactants for the synthesis of ammonia. The operating conditions required in the reactor are 200400C and 240260atm. Design engineers have two approaches to take N2H2 mixture to elevated pressures, (i) using a single-stage polytropic compressor, (ii) using a four-stage polytropic compressor (with intercoolers) with the same compression ratio of 4. The polytropic index is 1.282 , and compressor mechanical efficiency is 0.8 . The inlet flow rate is 2.5Sm3/s. The feed is at 1.5 atm and 15C. Polytropic efficiency will take care of the heat losses involved in the process. Consider polytropic efficiency as 0.95 (a) Find the exit temperature and power requirement for compressing the feed mixture to 256 atm using a single-stage polytropic compressor and compare it with isothermal compression. (b) Find the exit temperature and power requirement of each stage for compressing the feed mixture to 256 atm using a four-stage polytropic compressor (with intercoolers). (c) Comment on the viable design that can be recommended based on your calculationsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


