Please help!
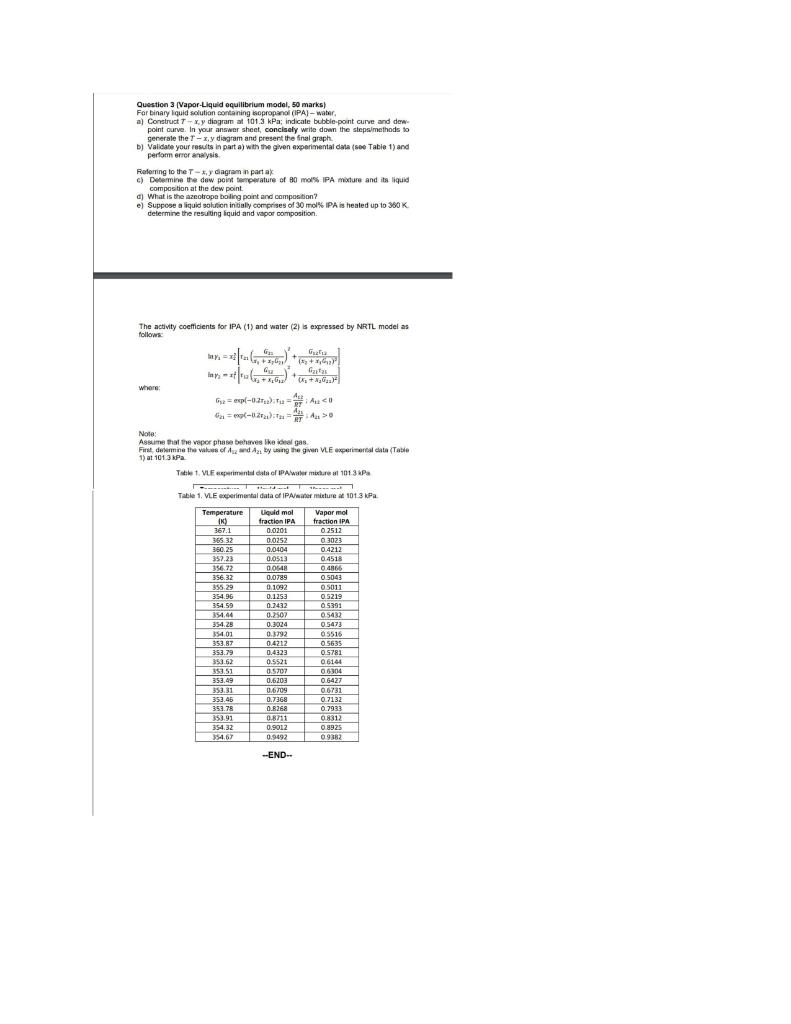
Qusstion 3 [Vapor-Liquid equilibrium model, 50 marks) For binary liquid solution contaning kopropanol (IPA) - water. a) Construct Txiy diagram at 101.3kPa; indicate bubble-point curve and dewpoint ourve. In your answer sheet, concisely write down the stepalmethods to generale the Tx,y diagram and present the final graph. b) Validate your results in part a) with the given experimental dats (soe Table 1) and perfam errer anelysis Feflering to the Tx,y dagram in part ak c) Determine the dew point terperature of 80 mons IPA mixure and ita liquid composition at the dew point. d) What is the azeotrope boling point and composition? e) Suppose a liquid solulion intialy comprises of 30mol IPA is healed up io 360K. determine the restiting liquid and vapor composition. The activity coefficients for IPA (1) and water (2) is expressed by NRTL model as follons. ln1=x22[r21(x1+x1G21G12)2+(t2+x1G12)212r12ln1=x2r13(x2+x112G21)2+(x2+x2G22)2G22t2! whare: 12=exp(0.2r22);r12=DVA22;A110 Note: Ascime that the vace phase behmoes tike ibeal gas Finct desaimine the vakows of A2L and A21 by wing the gien VLE axperimental eata (Table 1) at 101.3kPa. Tstik 1. M.F eperiment data of IPA waler mixtare at 101.3kPa ble 1, MLE experimental data of IPANwaner micture at 101.3WPa -END-- Qusstion 3 [Vapor-Liquid equilibrium model, 50 marks) For binary liquid solution contaning kopropanol (IPA) - water. a) Construct Txiy diagram at 101.3kPa; indicate bubble-point curve and dewpoint ourve. In your answer sheet, concisely write down the stepalmethods to generale the Tx,y diagram and present the final graph. b) Validate your results in part a) with the given experimental dats (soe Table 1) and perfam errer anelysis Feflering to the Tx,y dagram in part ak c) Determine the dew point terperature of 80 mons IPA mixure and ita liquid composition at the dew point. d) What is the azeotrope boling point and composition? e) Suppose a liquid solulion intialy comprises of 30mol IPA is healed up io 360K. determine the restiting liquid and vapor composition. The activity coefficients for IPA (1) and water (2) is expressed by NRTL model as follons. ln1=x22[r21(x1+x1G21G12)2+(t2+x1G12)212r12ln1=x2r13(x2+x112G21)2+(x2+x2G22)2G22t2! whare: 12=exp(0.2r22);r12=DVA22;A110 Note: Ascime that the vace phase behmoes tike ibeal gas Finct desaimine the vakows of A2L and A21 by wing the gien VLE axperimental eata (Table 1) at 101.3kPa. Tstik 1. M.F eperiment data of IPA waler mixtare at 101.3kPa ble 1, MLE experimental data of IPANwaner micture at 101.3WPa -END







