Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE HELP THANK YOU! (use the chart below) find the pH if E=-0.70 V Given the following reactions: Anode: NO3- + 2H+ + e- -->
PLEASE HELP THANK YOU! (use the chart below) 

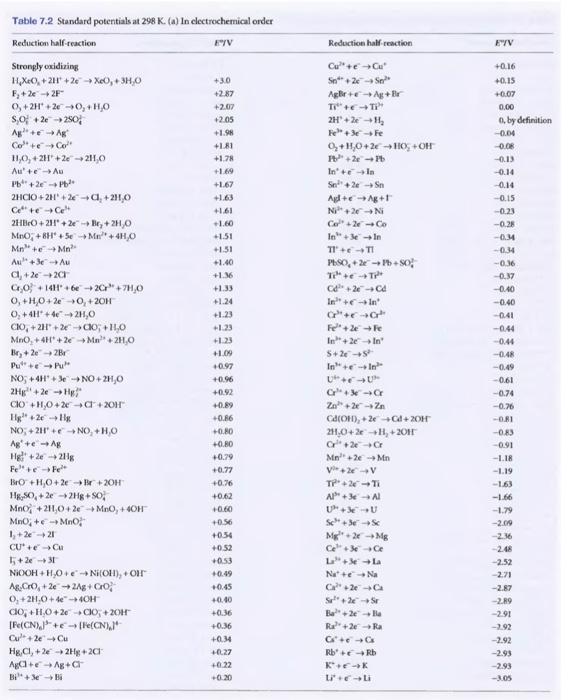
find the pH if E=-0.70 V
Given the following reactions: Anode: NO3- + 2H+ + e- --> NO2 + H2O Cathode: 2H+ + 2e---> H2 Redox: 2NO2(g) + 2 H20 (1) --> 2NO3-(aq) + 2H+(aq) + H2(g) [0.15 atm] Conditions: atm] [0.1 M] atm] [0.18 [0.67 Find the pH, if E = -0.70 V. - Table 7.2 Standard potentials at 298 K. (w) In clectrochemical order Reduction half-reaction HY Reduction half reaction EYV +30 +2.87 +2.07 +2.05 +1.9 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 -1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +1.24 1.23 Strongly oxidizing H.X(+2 +26X0,+ 3H,0 F,+2 +2F +2H4+2 -0,+HO SO +2e-250 Ag + Col + Col 11,0, +2 +2e211,0 Au' Au Pb+2 - 2HCIO+2H +26-0, +2310 Cette - 2HBO +211 +26-le,+ 2HO MnO +8H* +56 Mei +4H,O Mn + + Mn Au +36-A a, +2e-20 6,0 +1441 +61 +26+7HO O, +H.0+2e 0, +20H O, +41 + 42H,0 CIO, +231 +261 +11,0 MnO, + 4H+26+Mn +24,0 Br, +2 +2B Pue - NO, +4H'.JeNO2H,O 2H-2e - H CHO + HNO + 2x + 3OH High.2- NO + 2H+ NO +HO Agite A8 Hel+ 22116 Feel BO+H,O+2 + + 2OH HR.SO, +2 +2H8+SO, Mno, +211,0 +26+MnO, +4OH MnO, +6 +MnO 1, +2 +21 CUC 1+2+31 NOOH+H,00 NOHT), + oir A&CO, +2 +2Ag+GO O, +2,04e-OH co; +H.0+26 - 00,+2017 [Fe(CN).]+[C" Cu +2 Cu Hg,CI,+24 2Hg+20 Aga Ag+a Bill +0.23 +1.00 +0.97 +0.96 +0.92 -0.89 -0.86 -0.80 +0.80 +0.29 -0.77 +0.76 +0.62 Cu + Cu Sn" 26 - Agr+A+Bv TI" 2H'le Fee - Fe +140+26HOOH +2 - In In Sne-Sn Alt-Ag+ Ni+2N C2-Co Inse-In TITI PhSO, +- so: T + - C24 - Cd In-In C++ Fe +2 + In +2 In 5+24+ In-In UU G- 2+2 - CAOH), +2 +64 + 2OH 24.0+2+H+2011 G+ 2CE M2 - Men V2V T2-T AP + 3 + Al UPU SC3- Mg2Mg Ce+36 CE LLA Nae Na CH2-C S2-S E2-le Ba" + 2 + Ba G+ + Rb Rb KEK ti -0.16 +0.15 -0.07 0.00 o by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.28 -0.44 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41 -0.44 -0.44 --048 -0.49 -0,61 -0.74 -0.76 -0.81 -0.83 -0.91 -LIB -119 -1.63 -1.66 -1.79 -2.09 -2.16 -2.48 -2.52 2.71 -2.87 -2.89 -2.91 -2.92 -2.92 -2.93 -2.93 -3.05 +0.56 +0.54 +0.52 +053 +0.45 -0.40 +0.36 +0.36 +0.34 +0.27 -0.22 -020 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


