Question: Please help. The other question from chegg are not the same, so please do not repost another solution. For a solid solution consisting of two
Please help. The other question from chegg are not the same, so please do not repost another solution.
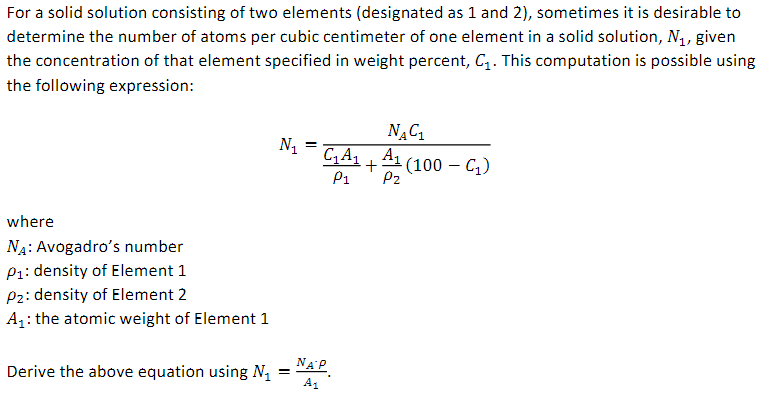
For a solid solution consisting of two elements (designated as 1 and 2), sometimes it is desirable to determine the number of atoms per cubic centimeter of one element in a solid solution, N1, given the concentration of that element specified in weight percent, C1. This computation is possible using the following expression: N1=1C1A1+2A1(100C1)NAC1 where NA: Avogadro's number 1 : density of Element 1 2 : density of Element 2 A1 : the atomic weight of Element 1 Derive the above equation using N1=A1NA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


